-
Meet researcher Saad Kenderian: Using regenerative cell therapy to recognize and attack cancer

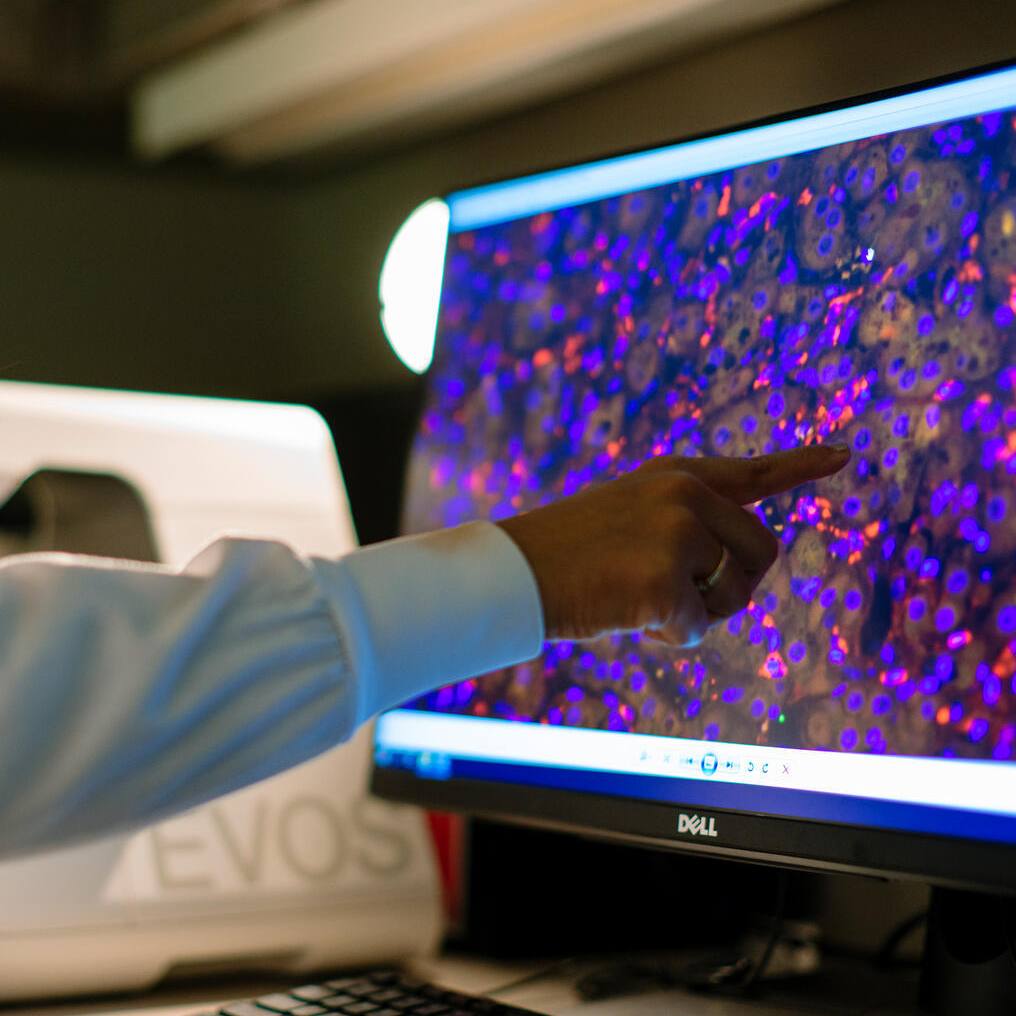
For as long as he can remember, Saad Kenderian, M.B., Ch.B., wanted to be a physician. Nothing could blunt his resolve –not even when exploding bombs and trappings of war forced the medical school in his hometown of Baghdad, Iraq, to close briefly. It is with that same determination he conducts Mayo Clinic research into chimeric antigen receptor (CAR) T-cell therapy, a regenerative therapy which unleashes the immune system to attack cancer.

“With CAR T, we are on the verge of discovering the potential of immune cells. The results that we are seeing are truly unprecedented, especially in B-cell leukemias and lymphomas. Some patients who really have no other hope are going into complete remission,” says Dr. Kenderian. “Through our research, we are discovering new CAR T products with fewer side effects. These new products may allow us to expand treatment to some blood cancers and solid tumors.”
The key hurdles to bringing CAR T-cell therapy to more patients are cost and access. It’s expensive and there are long waits for clinical trials. Dr. Kenderian’ s team is working collaboratively with Mayo Clinic Center for Regenerative Medicine, Mayo Clinic Cancer Center and the Mayo Clinic Department of Laboratory Medicine and Pathology’s Immune Progenitor and Cell Therapeutics Laboratory (IMPACT) lab to manufacture the cancer fighting cells in house and then ramp up production. Currently, Mayo uses commercial grade CAR T-cells engineered by an outside company.
“Making CAR T-cells in house could address issues of cost, access and innovation,” says Dr. Kenderian. “We can make the cells at a fraction of the cost, and we can increase production on our own timeline to increase availability and access to clinical trials. If an individual CAR T doesn’t work for a patient, we can go back and manufacture it according to each patient’s individual needs.”
Mayo Clinic Center for Individualized Medicine supports research into new CAR T products.
Fighting cancer with genetically engineered cells
CAR T-cell therapy seeks to harness the power of the immune system by genetically modifying cells, equipping them with power to kill cancer. These synthetic cells act like a living drug that uses the body’s defense system to fight disease.
“This is a prime example of regenerative immune therapy. Immune system T-cells are taken from each patient and engineered with an artificial protein that supercharges them to recognize and attack cancer. A large number of these cells are then injected back into the body. It’s a therapy shaped to each patient,” Dr. Kenderian says.
CAR T-cell therapy may be used on lymphoma and leukemia patients whose cancer has returned twice and no longer responds to standard therapy.
Fascination with the immune system
Part of Dr. Kenderian’s dream was to practice medicine in the United States. He never envisioned his career would put him on a circuitous path to harnessing the body’s ability to restore form and function.
After completing his residency at Michigan State University McLaren Hospital, he came to Mayo Clinic for a fellowship in hematology and oncology. It was during that time he grew fascinated with the power of the immune system and the potential that a patient’s body could fight disease.
“Tapping the immune system is perhaps one of the only therapeutic strategies that we can talk about as a potential cure (for cancer),” he says.
As part of his fellowship, Dr. Kenderian studied under the pioneers of CAR T-cell therapy at the University of Pennsylvania. He returned in 2016 to help establish the CAR T therapy program at Mayo Clinic.
Mayo Clinic is a leader in advancing CAR T-cell therapy
Mayo Clinic, which specializes in treating rare and complex conditions, is one of a select few medical centers in the United States to offer CAR T-cell therapy in a clinical setting. Dr. Kenderian says research is advancing this innovative treatment in the following ways:
- The number of clinical trials involving CAR
T-cell therapy has doubled in the past year. - There are two newly approved drug options for
leukemia and lymphoma; a third product is expected to clear U.S. Food and Drug
approval in early 2020. - Biomanufacturing of CAR T products is rapidly
increasing.
Dr. Kenderian says there is still progress to be made in increasing access and in getting insurance companies to pay. The collaborative approach to scaling up CAR T cell manufacturing at Mayo, he believes, is an important step toward solving those challenges.
###