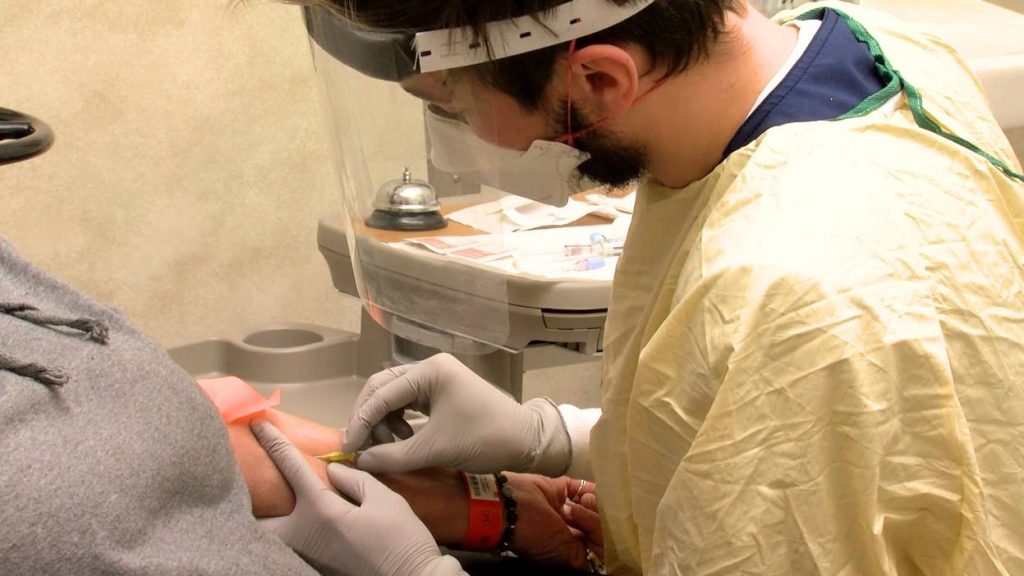
Mayo Clinic is treating patients with COVID-19 with monoclonal antibody treatments.
The Food and Drug Administration (FDA) issued an emergency use authorization to use bamlanivimab and casirivimab-imdevimab to treat confirmed COVID-19 in patients who have mild or moderate symptoms, and at a high-risk of disease progression and hospitalization. The treatments are given in the outpatient setting at infusion centers across Mayo Clinic's locations in the Midwest.
Dr. Raymund Razonable, a Mayo Clinic infectious diseases expert, discusses how the treatment works, who is eligible to receive it and why testing for early detection of COVID-19 is so important.
Watch: Dr. Raymund Razonable discusses monoclonal antibody treatments for COVID-19.
Journalists: Broadcast-quality soundbites are in the downloads at the end of the post along with b-roll video of a Mayo Clinic infusion center. Please courtesy: "Raymund Razonable, M.D. / Infectious Diseases / Mayo Clinic"
Who is eligible for the treatment?
The monoclonal antibody treatments help keep patients out of Mayo Clinic hospitals and decrease the severity of the disease. So far, Mayo Clinic has infused over 400 patients.
"The FDA has given emergency use authorization only for high-risk individuals. Those individuals are 65 years and older; have underlying conditions, such as diabetes or chronic heart disease, as well as those who have a compromised immune system, such as, patients with cancer and those who have undergone transplantation; those who are receiving high doses of steroids or other immunosuppressive drugs," says Dr. Razonable.
Dr. Razonable explains that a team meets every day to identify patients who are diagnosed with COVID-19, and determine who is in the high-risk category and who is eligible for the infusion treatment. The team then calls each eligible patient to offer the treatment and explain the process.
How it works
Patients receive one dose of this treatment through outpatient IV infusion. Treatments last around 2½ hours. Dr. Razonable says it's important for people to know that the treatment is not metabolized in the kidneys or liver, and is not expected to interfere with other patient medications.
"Monoclonal antibodies are basically antibodies that will attach to the spike protein of the SARS-CoV-2 virus (the virus that causes COVID-19). What it does is once it attaches to that spike protein, the virus cannot attach itself to the cells, thereby limiting the spread of the infection and preventing it from progressing further," explains Dr. Razonable.
Early testing key to treatment
"To be effective, this drug has to be given early, so that the protein of the virus can be bound to this antibody so that it prevents the infection from progressing," says Dr. Razonable.
"Many of the patients that we call, they just feel OK. They don't feel bad. It's just mild illness for them. But they are in the high-risk group category, meaning they belong to the group that is at high-risk of the illness progressing in the next several days. We want to catch them before they get worse, and that's been one of the most common reasons as to why some patients do not accept the drug because they thought that they don't need it because they feel fine," explains Dr. Razonable.
However, there does come a point when it's too late to give the drug. Dr. Razonable says the treatment no longer will be effective once patients require oxygen supplementation, if they develop shortness of breath, or their oxygen saturation is low. Testing to detect the infection and start treatment as early as possible in the course of the disease is important.
"Since we are really time-limited for the effectiveness of this drug, we wanted to catch them within the first seven to 10 days of illness. So if they get tested sooner, the sooner we know that they are eligible, the sooner that it can, that they could get this drug to prevent them from progressing further. So we emphasize the importance of early testing because that's going to be your entry point to be considered for this drug. You have to have a positive test," says Dr. Razonable.
Related posts:
- "COVID-19 experimental antibody treatment reaches patients"
- "Mayo Clinic innovations to ease burden of COVID-19 surge on hospitals"
Learn more about: Tracking and trending COVID-19.
Information in this post was accurate at the time of its posting. Due to the fluid nature of the COVID-19 pandemic, scientific understanding, along with guidelines and recommendations, may have changed since the original publication date.
For everyone's safety, Mayo Clinic has strict masking policies in place. Anyone shown without a mask was recorded prior to COVID-19 or recorded in an area not designated for patient care, where social distancing and other safety protocols were followed.
For more information and all your COVID-19 coverage, go to the Mayo Clinic News Network and mayoclinic.org.







