Although there is only one approved by the U.S. Food and Drug Administration (FDA) to treat coronavirus disease 2019 (COVID-19), many medications are being tested.
This article is written by Mayo Clinic Staff.
_______________________________________
The FDA has approved an antiviral drug called remdesivir (Veklury) to treat COVID-19 in adults and children who are age 12 and older. Remdesivir may be prescribed for people who are hospitalized with COVID-19. It's given through a needle in the skin (intravenously). The FDA has granted an emergency use authorization for the rheumatoid arthritis drug baricitinib (Olumiant) to treat COVID-19 in some cases. Baricitinib is a pill that seems to work against COVID-19 by reducing inflammation and having antiviral activity. The FDA states baricitinib may be used in combination with remdesivir in people who are hospitalized with COVID-19 who are on mechanical ventilators or need supplemental oxygen.
Researchers are studying other potential treatments for COVID-19, including:
- Antiviral drugs. In addition to remdesivir, other antiviral drugs being tested include favipiravir and merimepodib. Studies have found that the combination of lopinavir and ritonavir isn't effective.
- Dexamethasone. The corticosteroid dexamethasone is one type of anti-inflammatory drug that researchers are studying to treat or prevent organ dysfunction and lung injury from inflammation. Studies have found that it reduces the risk for deaths by about 30% for people on ventilators and by about 20% for people who needed supplemental oxygen.The U.S. National Institutes of Health has recommended this drug for people hospitalized with COVID-19 who are on mechanical ventilators or need supplemental oxygen. Other corticosteroids, such as prednisone, methylprednisolone or hydrocortisone, may be used if dexamethasone isn't available. Dexamethasone and other corticosteroids may be harmful if given for less severe COVID-19 infection.
- Anti-inflammatory therapy. Researchers study many anti-inflammatory drugs to treat or prevent dysfunction of several organs and lung injury from infection-associated inflammation.
- Immune-based therapy. Researchers study the use of a type of immune-based therapy called convalescent plasma. The FDA has granted emergency use authorization for convalescent plasma therapy to treat COVID-19. Convalescent plasma is blood donated by people who've recovered from COVID-19. It is used to treat people who are ill with COVID-19 in the hospital.Researchers also study other immune-based therapies, including mesenchymal stem cells and monoclonal antibodies. Monoclonal antibodies are proteins created in a lab that can help the immune system fight off viruses. Three monoclonal antibody medications have received emergency use authorization from the FDA. One medication is called bamlanivimab and the second medication is a combination of bamlanivimab and etesevimab. The third medication is a combination of two antibodies called casirivimab and imdevimab. All three drugs are used to treat mild to moderate COVID-19 in people who have a higher risk of developing serious illness due to COVID-19. Treatment consists of a single intravenous infusion given in an outpatient setting. To be most effective, these medications need to be given soon after COVID-19 symptoms start and prior to hospitalization.
- Drugs being studied that have uncertain effectiveness. Researchers study amlodipine and losartan. But it's not yet known how effective these drugs may be in treating or preventing COVID-19. Ivermectin and famotidine aren't likely to be beneficial in treating COVID-19.
- Hydroxychloroquine and chloroquine. These malaria drugs were authorized for emergency use by the FDA during the COVID-19 pandemic. However, the FDA withdrew that authorization when data analysis showed that the drugs are not effective for treating COVID-19. They can also cause serious heart problems.
- Drugs to prevent COVID-19. Researchers are studying drugs to prevent COVID-19 before and after exposure to the virus.
It's not known if any of these will prove to be effective against COVID-19. It's critical to complete medical studies to determine whether any of these medications are effective against COVID-19.
Don't try these medications without a prescription and your doctor's approval, even if you've heard that they may have promise. These drugs can have serious side effects. They're reserved for people who are seriously ill and under a doctor's care.
_________________________________________
For the safety of its patients, staff and visitors, Mayo Clinic has strict masking policies in place. Anyone shown without a mask was either recorded prior to COVID-19 or recorded in a nonpatient care area where social distancing and other safety protocols were followed.
Information in this post was accurate at the time of its posting. Due to the fluid nature of the COVID-19 pandemic, scientific understanding, along with guidelines and recommendations, may have changed since the original publication date.
For more information and all your COVID-19 coverage, go to the Mayo Clinic News Network and mayoclinic.org.
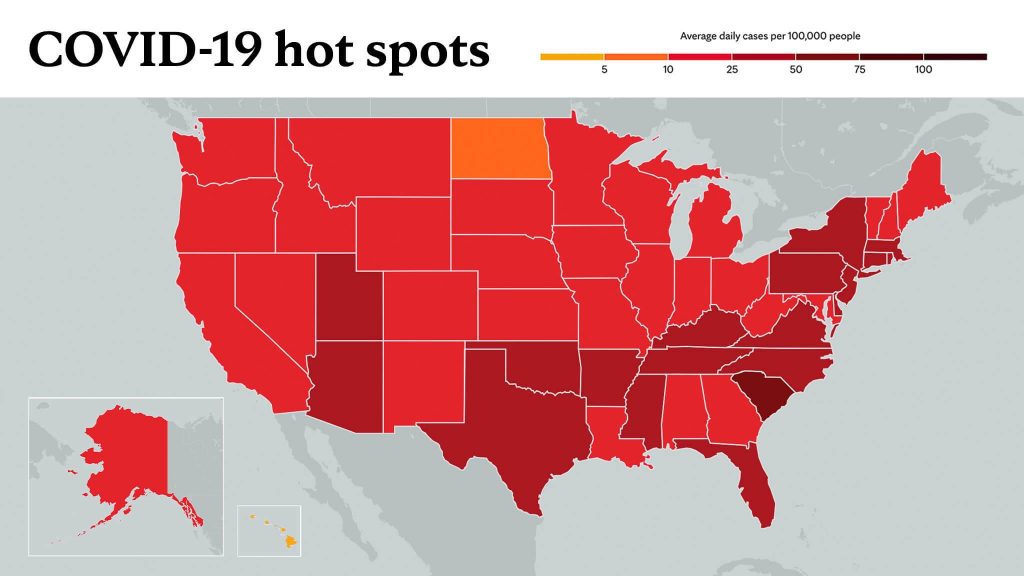
Learn more about tracking COVID-19 and COVID-19 trends.