The COVID-19 pandemic has brought public awareness to vaccines and how vaccines work. A vaccine is any agent that causes the immune system to remember a specific disease-causing entity, thereby preventing future infections. In the case of COVID-19, that's a coronavirus.
At Mayo Clinic, decades of research have led to development of a new vaccine platform — a single-cycle adenovirus nasal vaccine — that is now being tested in a phase 1 clinical trial for COVID-19.
“Single-cycle is particularly potent as a nasal vaccine, fighting SARS (severe acute respiratory syndrome) at its site of entry,” says Dr. Michael Barry, director of Mayo Clinic’s Vector and Vaccine Engineering Laboratory.
On the Mayo Clinic Q&A podcast, Dr. Barry discusses the research behind vaccine development and the possibility of future applications for the new vaccine platform.
Watch: Dr. Barry discuss vaccine development:
Read the full transcript.
____________________________________________
For the safety of its patients, staff and visitors, Mayo Clinic has strict masking policies in place. Anyone shown without a mask was either recorded prior to COVID-19 or recorded in a nonpatient care area where social distancing and other safety protocols were followed.
Information in this post was accurate at the time of its posting. Due to the fluid nature of the COVID-19 pandemic, scientific understanding, along with guidelines and recommendations, may have changed since the original publication date.
For more information and all your COVID-19 coverage, go to the Mayo Clinic News Network and mayoclinic.org.
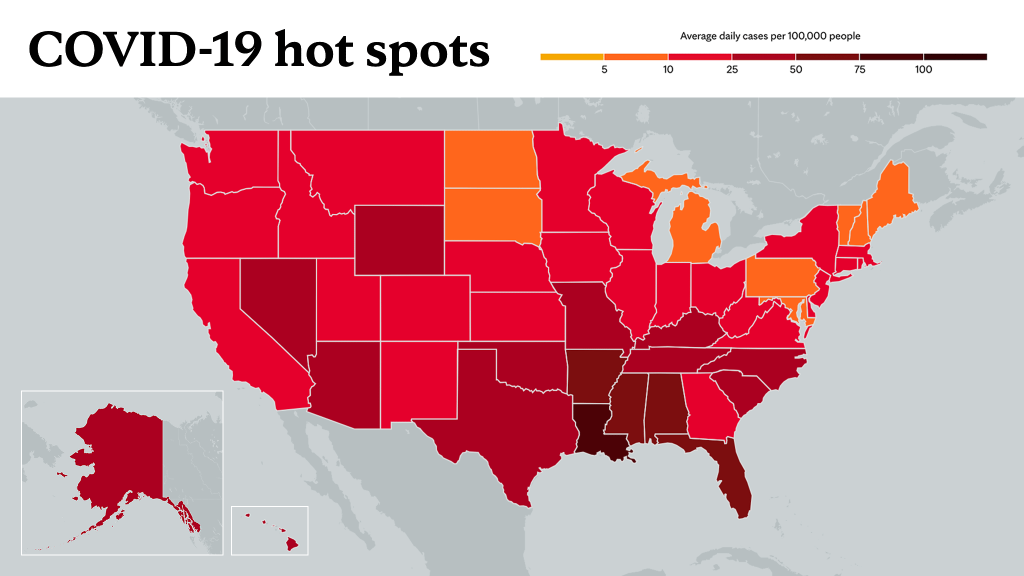
Learn more about tracking COVID-19 and COVID-19 trends.