"October is going to be a very exciting month in the U.S., regarding COVID-19 vaccines," says Dr. Gregory Poland, head of Mayo Clinic's Vaccine Research Group.
"On Oct. 14 the Federal Drug Administration is going to look at COVID-19 boosters for Moderna. On Oct. 15, (the FDA will review) boosters for Johnson & Johnsons' COVID-19 vaccine. And on Oct. 29, the FDA will look at extending emergency use for the Pfizer COVID-19 vaccine for children down to 5 years of age," says Dr. Poland. He adds that vaccinations of children could begin within a couple of weeks of the emergency use authorization.
Dr. Poland also says approval of Merck's antiviral COVID-19 pill is expected soon, too.
"We're excited about this because it's oral," says Dr. Poland. "The nice part about this is you can take it at home, and it fits with the same paradigm we already have in clinical medicine — treating influenza with an antiviral."
In this Mayo Clinic Q&A podcast, Dr. Poland also talks about waning immunities and the approval possibility of mixing and matching COVID-19 booster vaccines being approved. And he reminds women who are pregnant to get a COVID-19 vaccine.
Watch: Dr. Poland discusses continuing progress in the battle against COVID-19.
__________________________________
For the safety of its patients, staff and visitors, Mayo Clinic has strict masking policies in place. Anyone shown without a mask was either recorded prior to COVID-19 or recorded in a nonpatient care area where social distancing and other safety protocols were followed.
Information in this post was accurate at the time of its posting. Due to the fluid nature of the COVID-19 pandemic, scientific understanding, along with guidelines and recommendations, may have changed since the original publication date.
Research disclosures for Dr. Gregory Poland.
For more information and all your COVID-19 coverage, go to the Mayo Clinic News Network and mayoclinic.org.
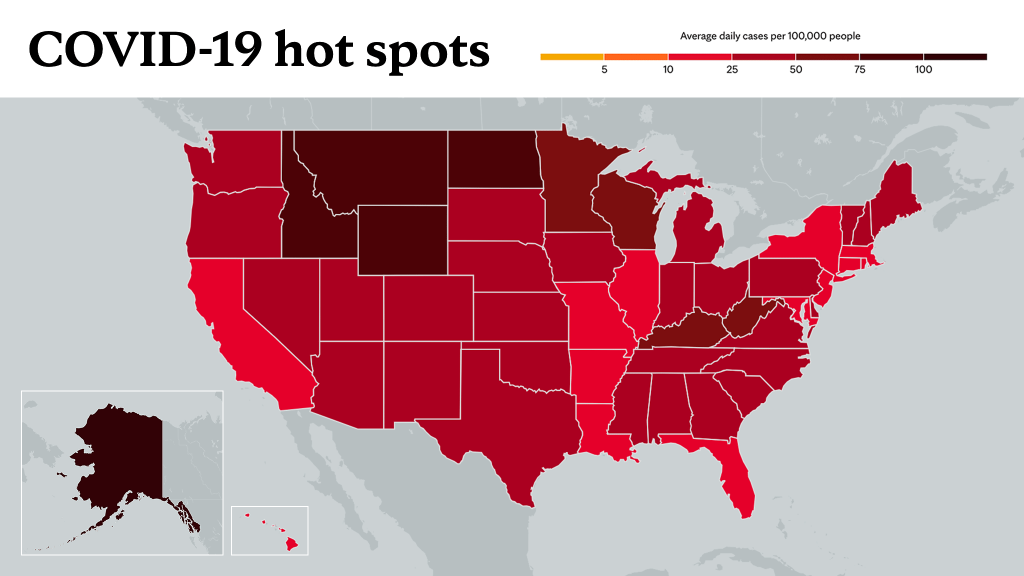
Learn more about tracking COVID-19 and COVID-19 trends.