-
Individualized Medicine
Mayo Clinic’s high-tech pursuit of precision alcohol addiction treatment

Millions of people worldwide are affected by alcohol use disorder, a debilitating addiction that can disrupt daily life and overall health. Alcohol use disorder is characterized by an inability to control drinking, a preoccupation with alcohol and continued use despite negative consequences.
According to the World Health Organization, 400 million people, or 7% of the world's population ages 15 and older, had an alcohol use disorder in 2019. Yet the primary prescribed medications, such as acamprosate, are only beneficial for a subset of patients.
To improve these outcomes, scientists at Mayo Clinic's Center for Individualized Medicine are pioneering the use of advanced research technologies to peel back the layers of this complex condition and understand the varying responses to treatments. Their goal is to develop personalized therapy strategies that are precisely tailored to each patient's genomic profile.
Building on this momentum, Mayo Clinic researchers recently conducted three pivotal studies focused on alcohol addiction, aiming to refine and personalize treatment strategies.
Discovery of biological markers
In their latest study, published in Brain, Behavior and Immunity, the team identified key biological markers that may predict individual responses to acamprosate, offering new insights into the mechanisms that influence treatment response.
"We've discovered that variations in the IL17RB gene can influence treatment outcomes with acamprosate, affecting important factors like how quickly someone might relapse and how long they can stay in recovery," says Ming-Fen Ho, Ph.D., a lead author and a stem cell biologist in the Department of Psychiatry and Psychology.
The discovery also provides insights into the underlying cause of the disorder and begins to suggest a potential path toward personalized treatments.
Pioneering a multi-omics approach

"Our work moves beyond traditional pharmacogenomics, which looks at how genes affect drug response, to a broader approach called pharmaco-omics," says Richard Weinshilboum, M.D., a lead author of the study and a pharmacologist in the Center for Individualized Medicine and Department of Molecular Pharmacology and Experimental Therapeutics. "We've used proteomics (proteins), genomics (genes) and transcriptomics (RNA) all together, representing a truly 'multi-omics' strategy that enhances our understanding of this disorder."
In this study, the researchers analyzed blood samples from 442 patients undergoing a three-month acamprosate treatment for alcohol use disorder. Using proteomics, a method that examines the entire set of proteins produced in cells, they identified 12 specific proteins that may help predict treatment response.
Genomic insights into treatment response
Expanding on these findings, the team then conducted a genome-wide association study to explore the data further. This approach involves examining a person's entire genome (DNA) to connect identified proteins with specific genetic variations. In this study, they focused on the IL17RB gene, known for its role in immune responses and inflammation.
They found that certain variants in this gene correlated with more favorable treatment responses. This indicates that these variants might serve as biomarkers to predict better outcomes with acamprosate.
Big vision for precision medicine
This research is part of a larger series of multi-omics projects aimed at unraveling the complexities of alcohol use disorder. Previously, the researchers used patient-derived induced pluripotent stem cells as a model system to study the effects of acamprosate and other potential therapeutics on the central nervous system.
Induced pluripotent stem cells are adult cells that have been reprogrammed to an early developmental state, allowing them to become any cell type, including neurons. This makes them a valuable tool for studying brain-related disorders.
Through this research, they identified a genetic variant linked to a hormone that increases with alcohol use and affects brain chemicals related to pleasure and reward.
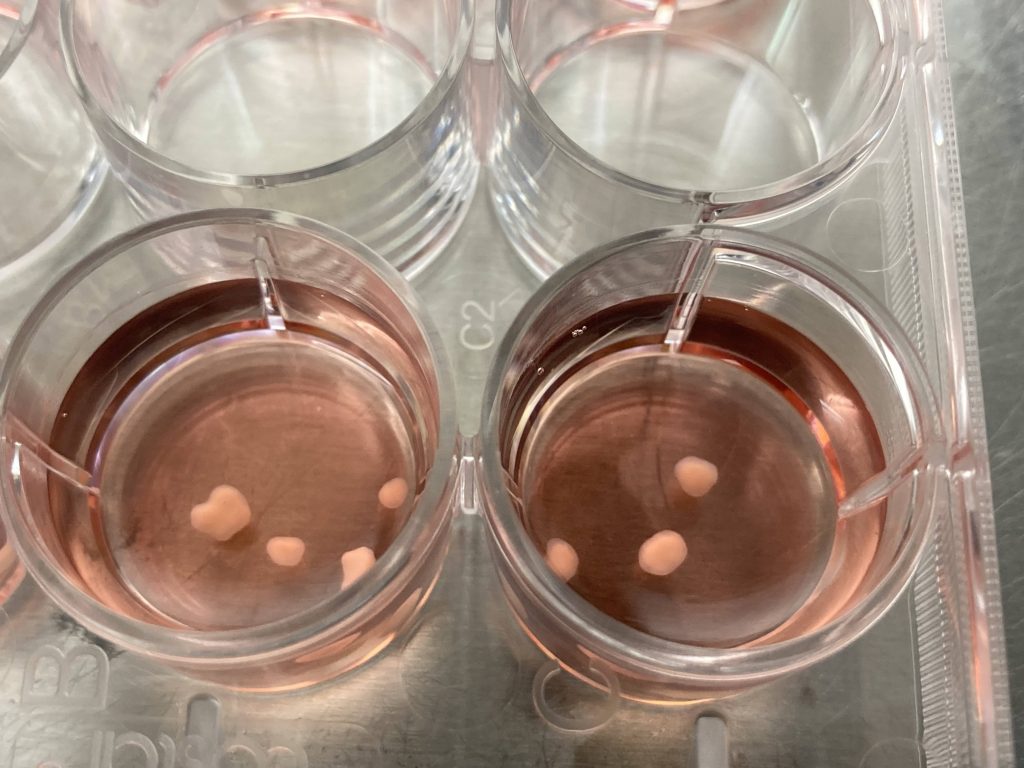
The team has also advanced their study of alcohol use disorder by developing brain organoids, which are lab-grown miniature 3D models of parts of the brain made out of human cells. These organoids replicate the brain's complex structure and functions and provide one type of realistic platform for studying the direct effects of alcohol on brain biology. Through this innovative approach, they identified a genetic variant that affects FGF21 protein levels and may influence the brain's system of pleasure and addiction.
“Each discovery has propelled the next, deepening our understanding of alcohol use disorder and refining potential treatment strategies,” Dr. Weinshilboum says. “This progress represents a new chapter in precision medicine and provides renewed hope for those affected by addiction disorders.”
Review the study for a complete list of authors, disclosures and funding.
Related Articles







