-
Individualized Medicine
Baby Oliver leaves legacy of genomic advances at Mayo Clinic
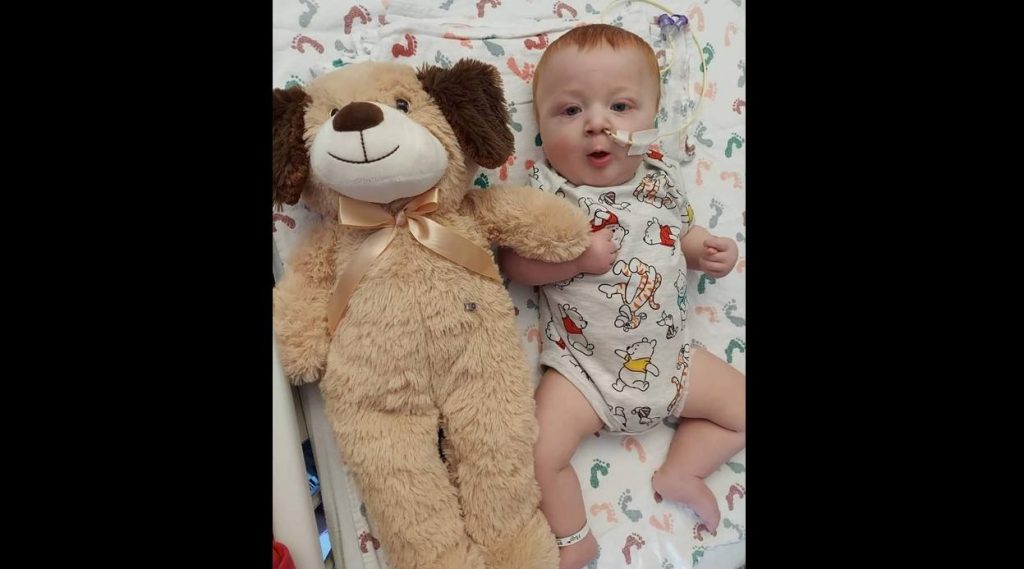
In his 18 months of life, Oliver Bates, with his soft red hair and big blue eyes, left an enduring legacy at Mayo Clinic. Oliver is remembered for his infectious smile and the love and resilience he radiated to all who knew him, even from the intensive care unit. Born with a rare and incurable genetic epilepsy disorder, he inspired a pioneering program designed to expedite genomic-related diagnoses and enhance definitive patient care.
"Oliver's life was a brief gift, but he left a mark deeper than his time with us," says his mother, Justine Bates, sitting in the comforting glow of purple lights in honor of her baby boy. Those lights, adorning the windows and a memory tree in her Minnesota home, symbolize epilepsy awareness. Close to her heart, Justine has a tattoo of Oliver's small handprint.
Oliver's father, Casey Bates, will always remember the quiet evenings spent holding his son after work. He proudly displays his own tattoo that reads "Seize the day," a subtle nod to his son's seizure condition and a reminder to cherish every day.
"He loved to laugh, and he had the best facial expressions. He was my snuggle bug," Casey says.
Oliver’s diagnostic journey
Oliver's health journey began when he was just five weeks old, when his first seizure led his parents to rush him to a local emergency room. The complexity of his case and the initial hospital's difficulty in diagnosing him led them to transfer Oliver to Mayo Clinic for specialized care. At Mayo, Oliver underwent a comprehensive series of tests, including exome sequencing, which specifically examines 20,000 protein-coding genes where many diseases originate. Although this sequencing technique can provide crucial insights into rare conditions, it can require time for detailed analysis and interpretation of the results.
While Oliver's initial tests did not reveal the cause of his ongoing seizures, the exome sequencing, which took nearly a month to process, finally helped his care team diagnose Oliver's condition: a rare form of epilepsy known as WWOX-related epileptic encephalopathy (WOREE syndrome). Although the diagnosis helped his care team guide Oliver's care, the condition currently has no cure. He passed away on March 10, 2022, surrounded by his loved ones.
A new era in genomics
Rare diseases affect 300 million people worldwide, yet only about 25 percent of patients ever receive a diagnosis, and often that diagnosis has no available treatments. Recent advancements in genomic technologies are beginning to offer a glimmer of hope. Clinicians are using these cutting-edge tools, which leverage artificial intelligence (AI) to scour volumes of data, to transform diagnostics and make it possible to identify and understand rare diseases more quickly and accurately than ever before. This is opening avenues for the development of more treatments.
Oliver inspires genomic advances
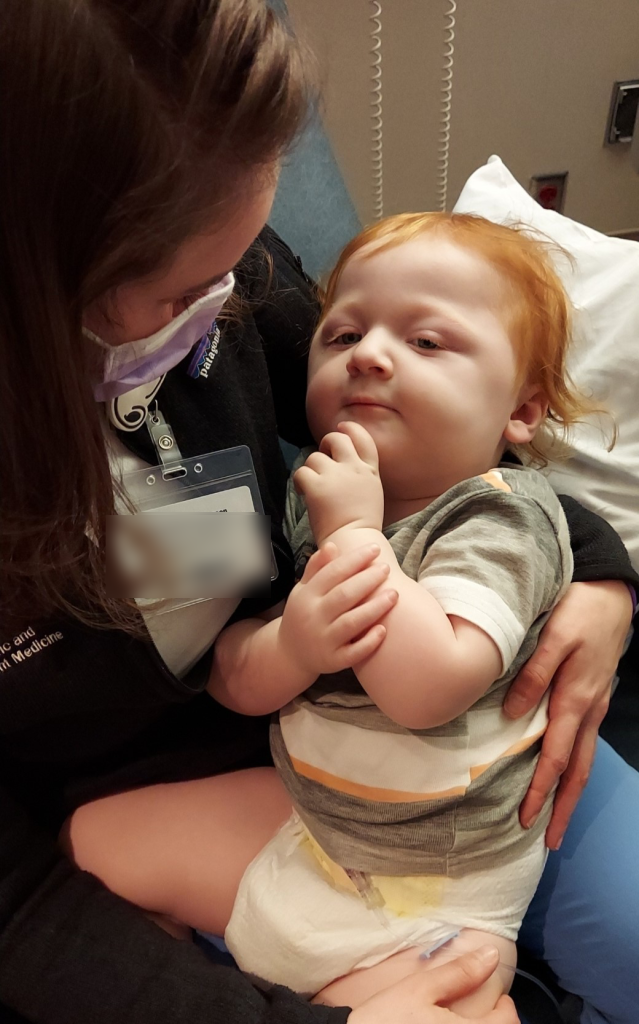
Witnessing firsthand the critical need for such advancements, Whitney Thompson, M.D., a key member of Oliver's care team, felt compelled to act, moved by Oliver's family's wait for a diagnosis. With support from the Department of Pediatric and Adolescent Medicine and the Center for Individualized Medicine, she launched an ultra-rapid whole genome sequencing program in Mayo Clinic's neonatal intensive care unit (NICU).
In this pioneering program, which is a collaboration with Rady Children's Institute for Genomic Medicine, clinicians use new sophisticated technology and AI to sequence a patient's 3 billion DNA base pairs in approximately 48 hours. This comprehensive analysis can identify even the most subtle genetic variants, though it may not always lead to a diagnosis.
"Rapid diagnoses can lead to lifesaving treatments in some cases, and while not every rare disease has a treatment, every diagnosis brings crucial information that can guide medical decisions and help families anticipate what’s next," says Dr. Thompson, who is completing her fellowship training in Neonatal Medicine, Clinical Genomics and Bioethics as part of the Clinician Investigator Training Program at Mayo Clinic.
Under this program, through the Mayo Clinic Department of Clinical Genomics with Brendan Lanpher, M.D., as practice chair, infants and children admitted to the Mayo Clinic's Eugenio Litta Children's Hospital in Minnesota undergo ultra-rapid whole genome sequencing if they meet specific criteria.
The program, launched at Mayo in June 2022, has since been expanded to some adults who exhibit symptoms suggestive of genetic disease, enabling them to receive the same rapid, comprehensive DNA analysis. To date, more than 300 infants, children and adults have been offered whole genome sequencing.
Dr. Lanpher hopes the program will open the door to more patients having access to comprehensive genetic testing.
"This is the future of medicine," Dr. Lanpher says. "I believe there are many patients with unrecognized or undiagnosed genetic diseases, and by finding and testing these patients early in the course of symptoms, we'll have the best chance at making a difference, finding a treatment and avoiding a diagnostic odyssey."
Introducing BabyFORce

Achieving a precision diagnosis is just the first step of a broader goal. Dr. Thompson and her team also have their sights set on rapid individualized therapeutics. She is collaborating with Laura Lambert, Ph.D., director of the Mayo Clinic Functional Omics Resource (FORce), along with Eric Klee, Ph.D., the Everett J. and Jane M. Hauck Midwest Associate Director of Research and Innovation at the Center for Individualized Medicine and Filippo Pinto e Vairo, M.D., Ph.D., director of the center's Program for Rare and Undiagnosed Diseases.
Together, they have initiated a first-of-its-kind program called BabyFORce, which uses AI to help clinicians identify individualized therapeutics for some of the smallest and sickest patients with genetic diseases in the neonatal intensive care unit.
"We’re tapping into an advanced AI platform that leverages a 'logic programming' approach, applying set rules to combine and analyze various biomedical data sources," says Dr. Lambert. "This could enable researchers to identify potential drug repurposing opportunities, generate hypotheses and uncover novel insights into disease mechanisms."
She says the approach could also reduce the time and cost associated with developing new therapies and provide hope for patients with limited treatment options.
Oliver's impact on pediatric tele-hospice
Oliver's impact extends beyond medical advancements. As his care unfolded during the challenging times of COVID-19, he also inspired Mayo's pediatric palliative care team to start a tele-hospice program. This ensured that children in hospice care could continue to receive compassionate support remotely.
"All of the providers who cared for Oliver learned so much from him and his family, including how helpful it was to have real-time video assessment of patients with challenging pain and symptoms during in-home hospice visits by nurses," says Christopher Collura, M.D., the Medical Director of Pediatric Palliative Care (ComPASS) at Mayo. "This inspired our team to formalize a tele-hospice program in order to streamline assessments by pediatric palliative medicine physicians for children enrolled with Mayo Clinic Hospice."
Honoring Oliver and welcoming his baby sister
Throughout Oliver's life and even in his final hours, Dr. Thompson and her team formed a tight, supportive relationship with Oliver and his family, providing comfort and care when it was most needed.

"Dr. Thompson was really there for us during the hardest time of our lives. And she has continued to support us after Oliver's death," Justine says. "Every year she joins us to honor his memory at our balloon release on Oliver's birthday."
The Bates family also reached out to Dr. Thompson for guidance on the potential for having another child.
WOREE syndrome, the genetic condition that affected Oliver, is a recessive disorder. That means both parents carry a copy of the mutated gene but usually show no symptoms. Each pregnancy has a 25% chance of the child inheriting two defective genes — one from each parent — which leads to the disease. Using these genetic insights, Dr. Thompson helped guide the Bates family to the subspecialists who helped them choose a plan that would increase the likelihood of a healthy pregnancy unaffected by the same genetic condition that Oliver had.
In May, Oliver's legacy was further celebrated with the birth of his baby sister. Justine and Casey named her Whitney, in tribute to Dr. Thompson, reflecting their deep gratitude for the care and dedication Dr. Thompson gave to Oliver and their family.
"I am deeply touched by this gesture," Dr. Thompson says. "Oliver's story shows us that new beginnings can coexist with cherished memories to help provide healing and comfort."
Related Articles







