-

JAMA: One-Year Data for Transcatheter Aortic Valve Replacement in U.S. Patients
ROCHESTER, MINN — Study results of one-year data for more than 12,000 patients who had transcatheter aortic valve replacement (TAVR) in the United States show an overall one-year death rate of 23.7 percent and a stroke rate of 4.1 percent, according to a study published in the March 10 issue of JAMA.
“Transcatheter aortic valve replacement has become transformational for patients who need a new valve and are at high-risk for surgery or inoperable. 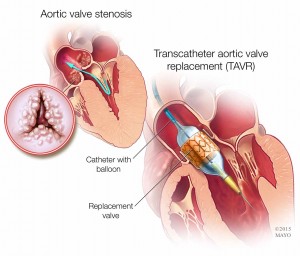 But we have been lacking long-term data for this group of patients who are considering this procedure,” says study lead author David R. Holmes, Jr., M.D., a Mayo Clinic interventional cardiologist. “Before this study, we only had 30-day information. This is a milestone and will help us better guide patients and learn as physicians.”
But we have been lacking long-term data for this group of patients who are considering this procedure,” says study lead author David R. Holmes, Jr., M.D., a Mayo Clinic interventional cardiologist. “Before this study, we only had 30-day information. This is a milestone and will help us better guide patients and learn as physicians.”
For the study, researchers used the Transcatheter Valve Therapies Registry, developed by the Society of Thoracic Surgeons and the American College of Cardiology, combining 12,182 TAVR patient procedures performed from November 2011 through June 2013 and linking to Centers for Medicare and Medicaid Services administrative claims for one-year data using direct Medicare patient identifiers (name and social security numbers).
Other important results from the study are:
- Median age of patients was 84 years old, and 52 percent of patients were female.
- 8 percent of patients were discharged directly to home.
- Thirty-day mortality was 7 percent.
- 4 percent of survivors were re-hospitalized only once, and 12.5 percent twice.
TAVR received U.S. Food and Drug Administration approval in 2011. It is used with increasing frequency for the treatment of severe aortic stenosis in patients who are at high or prohibitive risk for surgical aortic valve replacement, Dr. Holmes says.
Aortic valve stenosis happens when the valve narrows. The narrowing prevents the valve from fully opening, restricting blood flow from the heart into the aorta and then to the rest of the body. In TAVR, a minimally invasive procedure, physicians access the heart through a blood vessel in the leg or, less often, through an incision in the chest. A catheter is inserted through the access point, and the physician guides the catheter to the heart. Once in place, the replacement valve is passed through the catheter and positioned into place, and catheters are removed.
Other study authors and members of the TVT Registry are J. Matthew Brennan, M.D., M.P.H., David Dai, Ph.D., Sean O’Brien, Ph.D., and Sreekanth Vemulapalli, M.D., all of Duke Clinical Research Institute; John Rumsfeld, M.D., Ph.D., Denver VA Medical Center; Fred Edwards, M.D., University of Florida; John Carroll, M.D., and Fred Grover, M.D., both of University of Colorado; David Shahian, M.D., Massachusetts General Hospital; E. Murat Tuzcu, M.D., Cleveland Clinic; Eric Peterson, M.D., M.P.H., Duke University Medical Center; Ralph Brindis, M.D., M.P.H., University of California; and Michael Mack, M.D., Baylor Scott & White Health.
About Mayo Clinic
Mayo Clinic is a nonprofit organization committed to medical research and education, and providing expert, whole-person care to everyone who needs healing. For more information, visit http://mayocl.in/1ohJTMS, or https://newsnetwork.mayoclinic.org/.
MEDIA CONTACT: Traci Klein, Mayo Clinic Public Affairs, 507-284-5005, email: newsbureau@mayo.edu