-
Cancer
Bladder cancer: Research is driving new treatment options, better outcomes

Editor's note: May is Bladder Cancer Awareness Month.
Just 10 years ago, people with bladder cancer whose cancer had not spread and didn't respond to treatment often required bladder removal, says Mark Tyson, II, M.D., a Mayo Clinic Comprehensive Cancer Center urologic surgeon. "Now, we have a whole host of new drugs and clinical trials that allow us to offer more options," he says. Many of these options provide effective treatment while preserving the bladder. And clinical trials can provide additional options.
Most bladder cancers are urothelial carcinomas, which begin in the urothelial cells lining the inside of your bladder. Most are also non-muscle-invasive, meaning they have not spread beyond the bladder's muscle wall. While treatment depends on cancer type, grade, stage, the patient's overall health and other factors, Dr. Tyson says "We are, hopefully, going to be able to keep the disease at bay for the vast majority of people."
Dr. Tyson talks about new and developing bladder cancer treatments and what you should know when seeking bladder cancer care:
Individualized treatments and improved techniques are producing better outcomes
Newly approved drug combination
In 2023, the Food and Drug Administration (FDA) approved a two-drug combination of enfortumab vedotin and pembrolizumab (EV/pembro) to treat locally advanced or metastatic urothelial bladder cancer. The combination was previously only approved for the significant number of people who were ineligible for first-line treatment with a type of chemotherapy called cisplatin.
"Clinical trial results showed that EV/pembro was better than cisplatin and cisplatin-based combination chemotherapy in the first-line setting," says Dr. Tyson. Study results also highlighted a 55% reduction in disease progression or death compared to treatment with cisplatin-based chemotherapy, making EV/pembro the first treatment for urothelial bladder cancer to outperform chemotherapy when used as a first-line treatment.
"EV/pembro is now the new standard of care for patients with metastatic urothelial bladder cancer," says Dr. Tyson.
Single-port cystectomy (bladder removal surgery)
Cystectomy (bladder removal) might be recommended for muscle-invasive bladder cancer and for non-muscle-invasive bladder cancer that hasn't responded to other treatments or is likely to spread. It can be done with one large incision or several small incisions using minimally invasive surgery.
Dr. Tyson says Mayo Clinic is leading the way in refining a single-incision robotic surgery for bladder removal and reconstruction. This method shows potential for less pain, fewer postoperative complications and shorter hospital stays, although more research needs to be conducted.
Clinical trials testing new immunotherapy and less-invasive surveillance
Immunotherapy for treatment-resistant non-muscle-invasive bladder cancer
Immunotherapy, a drug treatment that helps your immune system fight cancer, is sometimes used to prevent the recurrence of non-muscle-invasive bladder cancer, especially when bladder preservation is a priority. A current standard-of-care immunotherapy is Bacillus Calmette-Guérin (BCG), a vaccine that uses weakened bacteria to stimulate the immune system to kill remaining cancer cells in the bladder. For high-grade, non-muscle-invasive bladder cancer that's unresponsive to BCG, a new immunotherapy called cretostimogene grenadenorepvec is on the fast track for FDA approval, says Dr. Tyson. Mayo Clinic is participating in a phase 3 clinical trial testing cretostimogene grenadenorepvec.
"We presented data at the annual meeting of the American Urological Association in 2024 showing promising results from a study conducted in patients with BCG-unresponsive, non-muscle-invasive bladder cancer," says Dr. Tyson. "As part of the trial, it's administered once a week for six weeks, and it's well tolerated, so patients don't have many side effects. Then we reassess them for three months, and if they've had a good response, they go on maintenance cretostimogene for three, six, nine, 12 and 18 months." Preliminary study results showed that out of 112 people who had completed the treatment course, 75.2% had a complete response, meaning that their cancer had not recurred. Eighty-three percent of complete responders who had one year of follow-up maintained their complete response beyond one year.
Urine test for non-muscle-invasive bladder cancer recurrence monitoring
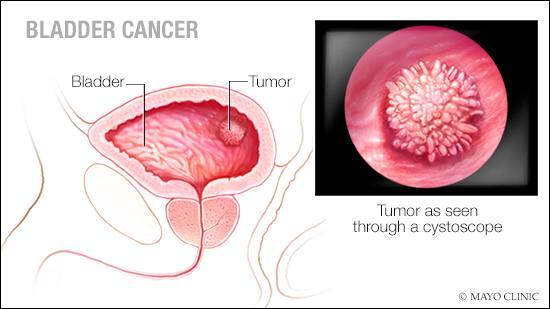
Because bladder cancer has a high recurrence rate and can progress from non-muscle-invasive to muscle-invasive disease during treatment, surveillance is important. Post-treatment monitoring often involves cystoscopy, a procedure where a scope is inserted through the urethra — the tube that carries urine out of your bladder — to examine the inside of your bladder.
Intravesical therapy, chemotherapy given directly into the bladder through a tube passed through the urethra, is an option for people with non-muscle-invasive bladder cancer. "Patients undergoing intravesical therapy generally need surveillance cystoscopy every three to six months for a couple of years, every six months for a couple of years, and yearly thereafter," says Dr. Tyson.
Cystoscopies can be uncomfortable and sometimes painful, and Dr. Tyson says a less-invasive surveillance method might become an option for people after non-muscle-invasive bladder cancer treatment. "One of the exciting studies we're doing at Mayo Clinic uses a urinary biomarker from a urine sample to identify recurrence without the need for cystoscopy," he says. The study aims to learn about people's preferences, comfort level and confidence in the urine test compared to cystoscopy.
Finding the most effective bladder cancer treatment for you
Choosing bladder cancer treatment is a personal decision. There are many factors to consider, including bladder preservation and quality of life. Dr. Tyson says it's important to find an attentive care team that treats a high volume of people with bladder cancer and has expertise in different surgical approaches to treatment.
"Try to find a healthcare professional willing to spend time with you to answer your questions," says Dr. Tyson. "If you feel rushed, that's not a good thing. If you feel like your care team isn't talking to you about all the options available, you're not getting a good opinion." He says learning about and considering all the treatment options is especially important if you want to preserve your bladder.
Dr. Tyson also recommends talking to your healthcare team about bladder cancer clinical trials, which might be beneficial if your cancer continues to advance after treatment. "People should look for care at centers that offer clinical trials, especially if they have non-muscle-invasive disease. Clinical trials are a good opportunity to get next-generation therapies."
Bladder cancer treatment and survival have improved drastically over the last decade. Dr. Tyson wants people with bladder cancer to know they have more options than ever before.
"If you have non-muscle-invasive bladder cancer, you're unlikely to die from your disease, and it's becoming more unlikely that you will lose your bladder," he says. "For muscle-invasive bladder cancer, the landscape has totally changed with EV/pembro. There's a lot of reason for hope. There's a lot of reason to think you can beat this disease."
This article first appeared on the Mayo Clinic Comprehensive Cancer Center blog.
Additional resources:
- Bladder cancer: What you should know about diagnosis, treatment and recurrence
- Bladder cancer patients require ongoing surveillance







