-

Exact Sciences Says Mayo Clinic is First Healthcare System to Offer Cologuard®
Stool-based DNA (sDNA) screening test for colorectal cancer to be available by prescription to patients
News Conference Advisory: An audio news conference was held this morning with representatives from Exact Sciences Corp. and Mayo Clinic.
Click here to listen or right click to download.
Click here for a transcript of the news conference.
MADISON, Wis., and ROCHESTER, Minn., — Exact Sciences Corp. (NASDAQ: EXAS) today announced that Mayo Clinic will be the first health system to offer Cologuard®, the first and only Food and Drug Administration (FDA) approved, noninvasive stool DNA screening test for colorectal cancer. Cologuard will be available to patients through their primary care physicians at Mayo Clinic.
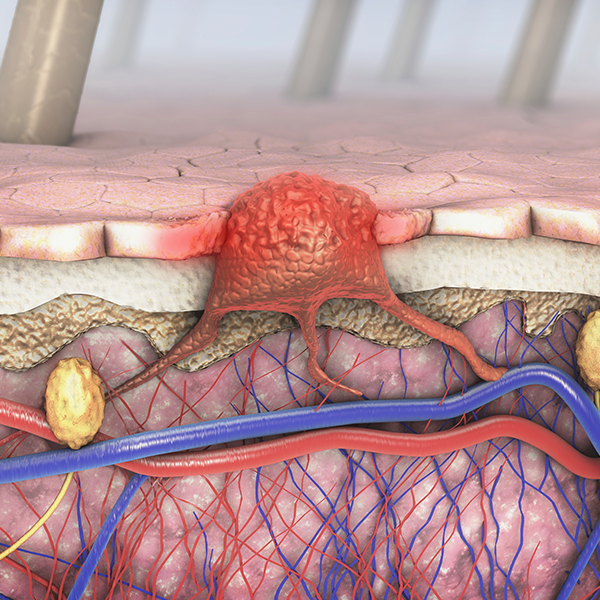
Available by prescription only, Cologuard offers people 50 years and older who are at average risk for colorectal cancer an easy to use screening test which they can do in the privacy of their own home. It is the first noninvasive screening test for colorectal cancer that analyzes both stool-based DNA and blood biomarkers to detect cancer and precancer. The Cologuard technology platform was co-developed by Exact Sciences Corp. and Mayo Clinic as part of a broad, exclusive collaboration.
“Cologuard represents a significant advancement in identifying colorectal cancer at its most treatable stage. We believe offering this new tool will promote patient and community public health and may move more patients to get screened earlier—a critical step in beating this prevalent and preventable cancer,” says Vijay Shah, M.D., chair of Mayo Clinic gastroenterology and hepatology.
MEDIA CONTACT: Brian Kilen, Mayo Clinic Public Affairs, 507-284-5005, newsbureau@mayo.edu
Journalists: Video is available in the downloads.
Colorectal cancer is highly preventable with screening. However, twenty-three million Americans between 50 and 75 are

not getting screened as recommended and, as a result, colorectal cancer remains the second-leading cancer killer in the United States. For those whose cancer is detected at an earlier stage, the five-year survival rate can be greater than 90 percent.
“Mayo Clinic’s adoption of Cologuard marks a significant step forward in providing patients access to this important new cancer screening technology,” said Kevin Conroy, president, CEO and chairman of Exact Sciences Corp. “We look forward to continuing our collaboration with the Mayo Clinic to develop a growing pipeline of diagnostic screening innovations for GI tract cancers.”
Cologuard is designed to detect DNA alternations and blood released from cancer and precancerous colon lesions. The test requires a physican order. After the physician orders Cologuard, the kit is mailed directly to the patient’s home. The patient then collects a stool sample in the Cologuard Collection Kit, and sends the kit back to the Exact Sciences lab for testing through a pre-paid mailer.
Patients learn of their results in as little as two weeks from their physician. Patients with a positive result will need to undergo colonoscopy.

“Low screening rates have long contributed to low survival rates for colorectal cancer, with more than 60 percent of all cases not detected until late stages of the disease,” said David Ahlquist, M.D., a Mayo Clinic gastroenterologist and co-inventor of the test. “I am hopeful that the test’s efficacy and convenience will result in improved detection and survival rates for colorectal cancer.”
For more information, visit www.CologuardTest.com or call 1-844-870-8870. More information on colon cancer and the importance of screening and early detection at http://www.beseengetscreened.com/. Visit www.exactsciences.com to sign up for the company’s eNewsletter.
Disclosure statement - David Ahlquist, M.D., is a co-inventor of the technology that has been licensed to Exact Sciences from Mayo Clinic. Under that licensing agreement, Mayo Clinic and Dr. Ahlquist share in equity and royalties. Revenue Mayo Clinic receives is used to support Mayo's not-for-profit mission in patient care, education and research.
###
About Exact Sciences Corp.
Exact Sciences Corp. (NASDAQ: EXAS) is a molecular diagnostics company focused on the early detection and prevention of colorectal cancer. The company has exclusive intellectual property protecting its noninvasive, molecular screening technology for the detection of colorectal cancer. Stool DNA technology is included in the colorectal cancer screening guidelines of the American Cancer Society and the U.S. Multi-Society Task Force on Colorectal Cancer. For more information, please visit the company's website at www.exactsciences.com, follow us on Twitter @ExactSciences or find us on Facebook.
About Mayo Clinic
Recognizing 150 years of serving humanity in 2014, Mayo Clinic is a nonprofit worldwide leader in medical care, research and education for people from all walks of life. For more information, visit 150 years.mayoclinic.org, mayoclinic.org, newsnetwork.mayoclinic.org.
Certain statements made in this news release contain forward-looking statements within the meaning of Section 27A of the Securities Act of 1933, as amended, and Section 21E of the Securities and Exchange Act of 1934, as amended that are intended to be covered by the “safe harbor” created by those sections. Forward-looking statements, which are based on certain assumptions and describe our future plans, strategies and expectations, can generally be identified by the use of forward-looking terms such as “believe,” “expect,” “may,” “will,” “should,” “could,” “seek,” “intend,” “plan,” “estimate,” “anticipate” or other comparable terms. Forward-looking statements in this news release may address the following subjects among others: statements regarding the sufficiency of our capital resources, our ability to secure favorable reimbursement rates from Medicare and other third-party payors, timing of our launch of a commercial product, our estimates of the available market size and our potential penetration, expected research and development expenses, expected general and administrative expenses and our expectations concerning our business strategy. Forward-looking statements involve inherent risks and uncertainties which could cause actual results to differ materially from those in the forward-looking statements, as a result of various factors including those risks and uncertainties described in the Risk Factors and in Management’s Discussion and Analysis of Financial Condition and Results of Operations sections of our most recently filed Annual Report on Form 10-K and our subsequently filed Quarterly Reports on Form 10-Q. We urge you to consider those risks and uncertainties in evaluating our forward-looking statements. We caution readers not to place undue reliance upon any such forward-looking statements, which speak only as of the date made. Except as otherwise required by the federal securities laws, we disclaim any obligation or undertaking to publicly release any updates or revisions to any forward-looking statement contained herein (or elsewhere) to reflect any change in our expectations with regard thereto or any change in events, conditions or circumstances on which any such statement is based.