-
Individualized Medicine
First hybrid gene therapy shows early promise in treating long QT syndrome
ROCHESTER, Minn. ― In a new study published in Circulation, Mayo Clinic researchers provide the first preclinical, proof-of-concept study for hybrid gene therapy in long QT syndrome, a potentially lethal heart rhythm condition.
Researchers demonstrated its potential therapeutic efficacy in two in vitro model systems using beating heart cells reengineered from the blood samples of patients with type 1 long QT syndrome. They targeted the whole KCNQ1 gene rather than specific LQT1-causative mutations, making this study applicable to all patients with type 1 long QT syndrome, regardless of their specific disease-causing variant.
The team consisted of Mayo Clinic researchers from Mayo Clinic's:
- Department of Cardiovascular Medicine.
- Department of Molecular Pharmacology and Experimental Therapeutics.
- Department of Pediatric and Adolescent Medicine.
- Center for Individualized Medicine.
- Center for Regenerative Medicine.
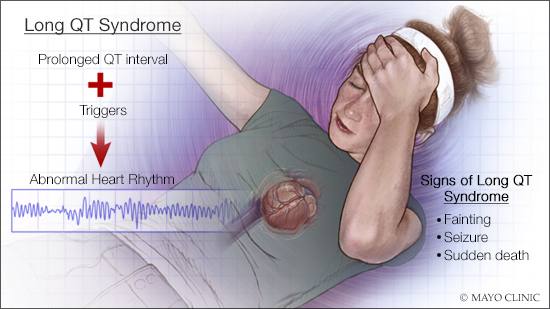
The prevalence of long QT syndrome is about 1 in 2,000. When untreated, high-risk patients have an estimated 10-year mortality of 50%.
Long QT syndrome is a genetic heart rhythm condition that can potentially cause fast, chaotic heartbeats. These rapid heartbeats might trigger people to suddenly faint. Some people with the condition have seizures. In some severe cases, long QT syndrome can cause sudden cardiac death. The most common subtype, type 1 long QT syndrome, or LQT1, is caused by pathogenic variants in the KCNQ1 gene.
"Gene therapy is an emerging area of interest for treating a variety of genetic heart diseases in general and long QT syndrome in particular," says Michael Ackerman, M.D., Ph.D., a Mayo Clinic genetic cardiologist and director of Mayo Clinic's Windland Smith Rice Comprehensive Sudden Cardiac Death Program. "We designed and developed the first suppression and replacement KCNQ1gene therapy approach for the potential treatment of patients with type 1 long QT syndrome." Dr. Ackerman is senior author of this study.
According to Dr. Ackerman, over the past two decades, substantial improvements have been made to manage long QT syndrome, but current therapies, such as beta blockers and defibrillators, a more invasive therapy, still have limitations, risks and an array of unwanted side effects.
Gene therapy is a technique that treats diseases caused by defective genes by altering genes in a patient's cells rather than using drugs or surgery. Genes contain DNA — the code that controls the body's form and function. Gene therapy replaces faulty genes or adds a new gene to try to treat a disease.
According to Dr. Ackerman in this case, this is the first time that hybrid gene therapy (simultaneous out with the old, in with the new) has been created for any form of genetic heart disease.
"If the therapeutic efficacy of this 'disease-in-the-dish' gene therapy trial with KCNQ1 can be replicated in a nonhuman, animal model of long QT syndrome, then suppression-replacement (hybrid) gene therapy may be a promising strategy for long QT syndrome in general and in theory almost any sudden death-predisposing autosomal dominant genetic heart disease," says Dr. Ackerman. "Of course, we still have a long way to go from nearly curing a patient's heart cells in the dish to effectively treating the whole person. Nevertheless, we are excited by this first critical milestone and look forward to the next step."
In addition to heart disease, researchers at Mayo Clinic's Center for Individualized Medicine are investigating an approach to replace and fix mutated genes in a wide range of genetic disorders.
Funding
This work was supported by Mayo Clinic's Windland Smith Rice Comprehensive Sudden Cardiac Death Program (MJA), the Dr. Scholl Foundation (MJA), Mayo Clinic's Center for Individualized Medicine High-Definition Therapeutics grant (MJA), and Mayo Clinic's Department of Molecular Pharmacology and Experimental Therapeutics training grant under National Institutes of Health award T32GM072474 (SMD and MJA).
Disclosures
Dr. Ackerman is a consultant for Abbott, Armgo Pharma Inc., Audentes Therapeutics, Boston Scientific Corporation, Daiichi Sankyo Co. Ltd., Invitae Corporation, LQT Therapeutics Inc., Medtronic, MyoKardia, and UpToDate Inc. Dr. Ackerman and Mayo Clinic are involved in an equity/royalty relationship with AliveCor Inc.
About Mayo Clinic
Mayo Clinic is a nonprofit organization committed to innovation in clinical practice, education and research, and providing compassion, expertise and answers to everyone who needs healing. Visit the Mayo Clinic News Network for additional Mayo Clinic news and Mayo Clinic Facts for more information about Mayo.
Media contact:
- Colette Gallagher, Mayo Clinic Public Affairs, newsbureau@mayo.edu
Related Articles







