-
Biotherapeutics
Regenerative medicine and diabetes treatment
Nearly everyone knows someone with diabetes — it's hard not to. In the United States, 1 in 3 adults and 1 in 6 children have high blood sugar, according to the National Institutes of Health.
After you eat, glucose is absorbed into your bloodstream and carried throughout your body. Insulin — a hormone made by beta cells in your pancreas — then signals your cells to take up glucose, helping your body turn the food into energy.
With diabetes, this process can go wrong in two basic ways: Type 1 diabetes results from the body's failure to produce insulin; type 2 diabetes occurs when there's plenty of insulin but the cells lose their ability to perceive its signal. In both cases, cells starve.
Living well with diabetes requires a lifelong commitment to monitoring blood sugar, eating properly, exercising regularly and maintaining a healthy weight. People with type 1 diabetes must also rely on insulin replacement therapy, usually through insulin injections. People with type 2 diabetes might need oral medication.
Still, every year, diabetes kills about 70,000 people in the United States and is a contributing cause in another 160,000 deaths each year, according to the Centers for Disease Control and Prevention.
Yasuhiro Ikeda, D.V.M., Ph.D., a molecular biologist at Mayo Clinic in Rochester, Minn., wants to change that.

After beginning his career as a veterinary feline specialist, Dr. Ikeda had to change course when he developed an allergy to his four-legged patients that made it impossible to be in a room with them. He turned his attention toward research and discovered that his interest in molecular virology had human as well as feline applications.
The story of one Mayo Clinic patient with diabetes turned Dr. Ikeda's attention back toward healing.
"He had to have his legs amputated, then went blind, then developed kidney disease," Dr. Ikeda recalls. "He couldn't walk, couldn't see, and one day he decided not to come in. He refused treatment and died a few days later. That story stays in my mind. That's why I'm here now, because this new technology may really change things."
And that's why Dr. Ikeda and his research team are screening hundreds of thousands of compounds to discover potential drugs to treat type 2 diabetes — drugs that don't lead to crashing blood sugar levels.
Dr. Ikeda — known as Ike to friends and colleagues — is also working toward developing a cure for type 1 diabetes. He plans to use gene therapy to prevent pancreatic beta cell loss in patients newly diagnosed with type 1 diabetes.
"We are almost there," he says.
Gene therapy for type 1 diabetes
People develop type 1 diabetes when their own immune system attacks and kills their pancreatic beta cells.
"The idea is simple: If we can suppress autoimmunity, we should be able to stop the disease," Dr. Ikeda explains.
As symptoms begin to show up, most patients experience what researchers call the honeymoon period, which typically lasts for several months after someone has been diagnosed with diabetes. During this time, many of their beta cells remain functional.
"If we can block the autoimmune response then," Dr. Ikeda says, "the beta cells can recover all by themselves."
Gene therapy uses DNA as a drug to fight disease. The interventional gene is packaged so it is deliverable to cells within a specific tissue in the body.
Dr. Ikeda has already perfected a gene delivery system that takes advantage of a special virus engineered to carry the interleukin-10 (IL-10) gene, a potent protein that can rein in excessive immune reactions. The virus is called AAV — short for adeno-associated virus.
AAV is special because it doesn't cause disease and doesn't replicate, which means it elicits only a mild immune response. AAV infects both dividing and nondividing cells and persists without integrating into the genome of the host cell. This gets around the complication of disrupting important chromosomal genes.
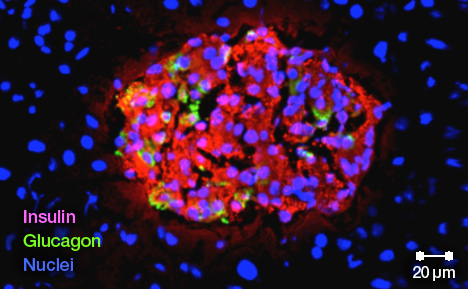
Dr. Ikeda's treatment turns the gene on only where concentrations of insulin are high — that is, inside beta cells. He has successfully used his system to prevent type 1 diabetes in animal studies.
Making more beta cells in diabetes
Blocking the diabetic autoimmune response will probably not be enough to cure type 1 diabetes all on its own, Dr. Ikeda acknowledges. He wants to develop a treatment that keeps the immune system from destroying what beta cells are left, while helping the body make more of them.
Two molecules may help make this possible. The first, glucagon-like peptide 1 (GLP-1), a gastrointestinal hormone, stimulates the pancreas to make more beta cells, which increases the amount of insulin released. The second, a version of glucokinase, stimulates beta cells to grow larger.
Dr. Ikeda is gearing up to test the safety and feasibility of this treatment in nonhuman primates in collaboration with researcher Melanie Graham, M.P.H., Ph.D., at the University of Minnesota in Minneapolis. He is also working with Stephen J. Russell, M.D., Ph.D., deputy director for translation at the Mayo Clinic Center for Regenerative Medicine in Rochester, to prepare for the next step — human gene therapy trials.
Combining gene therapy with stem cell therapy
For patients who have moved beyond their honeymoon period, who have few or no beta cells left, Dr. Ikeda is holding out one more option: healing patients with type 1 diabetes using stem cells created from their own healthy cells.
This approach to treating type 1 diabetes combines gene therapy and stem cell therapy. Healthy cells with helpful genes are introduced into affected tissues.
Regenerative medicine therapies like these may restore normal function to damaged or diseased tissues or organs. They may treat the underlying cause of a disease rather than only manage symptoms.
"I see regenerative medicine as the new surgery," says Dr. Russell, who is also the Richard O. Jacobson Professor of Molecular Medicine at Mayo Clinic.
Stem cells can divide and differentiate into multiple cell types to become parts of all kinds of tissues, such as nerves, muscles or blood. Naturally occurring sources of stem cells include placental or cord blood stem cells. Induced pluripotent stem (iPS) cells like the ones Dr. Ikeda is using are custom-designed for each patient.
Induced pluripotent stem cells are made by reprogramming the patient's own adult cells. The process for creating these stem cells begins by taking the patient's skin or blood cells and through a series of steps, inducing them to de-differentiate into pluripotent cells.
The iPS cells themselves can't be directly used for therapies. Not only would these cells be able to cause tumors, but they would also be unlikely to differentiate into beta cells on their own. They need to be coaxed to specialize into becoming functioning beta cells before being introduced into the pancreas.
The main advantage of Dr. Ikeda's combination therapy is that the new, introduced cells are not subject to organ rejection or availability because they come from the patients themselves, not a donor. The other advantage is the opportunity to give those cells a fighting chance of turning into productive beta cells by using gene therapy to turn on and express therapeutic proteins like IL-10, GLP-1 and the hyperactive form of glucokinase.
Dr. Ikeda's team has established a consistent recipe of sorts for making these glucose-responsive, insulin-producing cells using the skin cells of patients with diabetes. In collaboration with biochemist Jonathan Slack, Ph.D., of the University of Minnesota at Minneapolis, the team has also been able to regenerate human pancreatic islets — the regions of the pancreas that contain the beta cells — in preclinical studies.
Discovering new drugs for type 2 diabetes
In addition to fighting type 1 diabetes, Dr. Ikeda's group is screening hundreds of thousands of compounds in an effort to discover potential new classes of type 2 diabetes drugs.
Type 2 diabetes is usually controlled first through diet and exercise, which can improve the body's ability to use insulin properly. Often, though, lifestyle changes are not enough to control diabetes. When that's the case, the next step is oral medication.
Currently available diabetes medications stimulate the pancreas to ramp up insulin production. The body is still not using insulin as effectively as it should, but there is more of it to signal the body's cells to take up glucose.
Unfortunately, the drugs keep right on stimulating insulin secretion even as blood sugar levels drop. This can cause hypoglycemia, which can lead to confusion, clumsiness and fainting and, if uncontrolled, to seizures, coma and even death.
So in collaboration with pharmacologist Gunda Georg, Ph.D., director of the Institute for Therapeutics Discovery & Development, at the University of Minnesota, Dr. Ikeda is looking for new drug candidates that stimulate insulin secretion only in the presence of glucose.
Dr. Ikeda says he is grateful for the opportunity to pursue this research at Mayo Clinic. "I was lucky to end up here, working with the people I do," he says.
- Megan McKenzie