-
Knowledge of DNA repair mechanisms advances with new paper from Mayo Clinic scientists
ROCHESTER, Minn. ─ We humans like to think our DNA is well-protected in the nucleus of each cell. But it’s a hard life for the hard-working genetic code.
DNA can be damaged by a range of normal cellular activities, not to mention ultraviolet light and ionizing radiation. The bad news is that damage, such as DNA double-strand breaks, is constantly occurring. But the good news is the human body has so-called damage response proteins to fix it.
Now, thanks to a new publication by Mayo Clinic researchers, scientists know more about these damage response proteins and how they do their job.
“This basic research is aimed at understanding how DNA damage response proteins work,” explains Georges Mer, Ph.D., a Mayo Clinic biochemist. “We hope the knowledge gained from these studies might, in the long term, be beneficial from a therapeutic perspective, notably for cancer treatment.”
Double-strand breaks
Certain types of radiation, such as gamma rays and X-rays, can cause breaks in both strands of DNA. If it’s unfixable, the damage can trigger a cell to die. But Dr. Mer and team examined the way in which these breaks can trigger chemical signals to mobilize the DNA repair proteins.
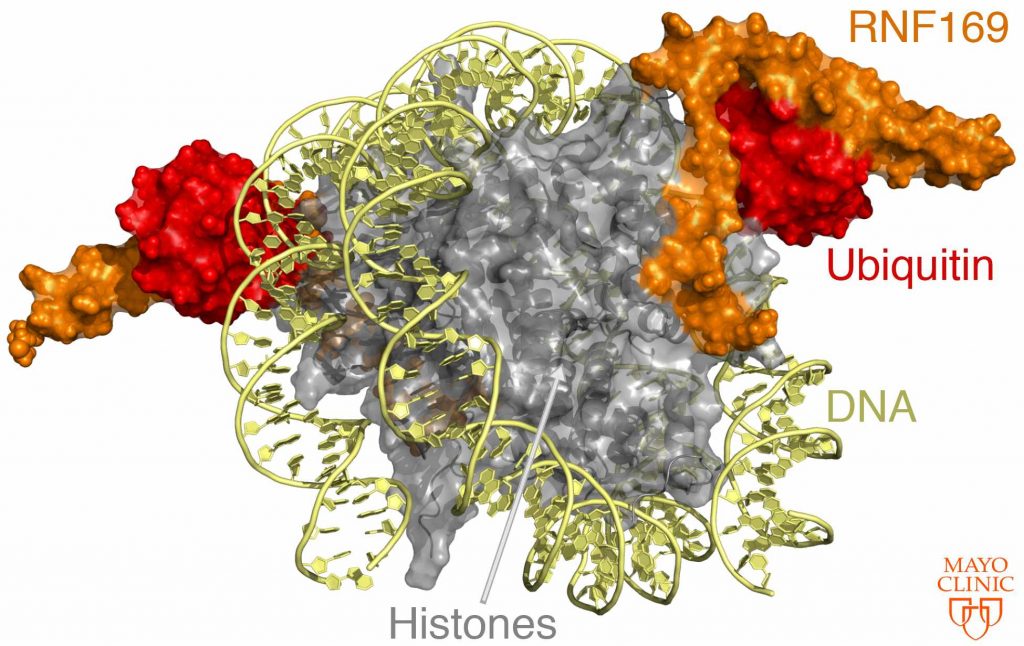
Within the nucleus of the cell, DNA is wrapped around proteins called histones. A package of eight histones and DNA is called a nucleosome. When a DNA double-strand break occurs, histones are chemically modified at different positions. The authors studied the modification that occurs when a molecule called ubiquitin attaches to a histone in response to the DNA break. They examined the attachment of ubiquitin on the H2A histone at two positions, and, when the call goes out, how the damage response protein attaches to the nucleosome.
“By deriving a detailed 3-D structure of [the damage response protein] RNF169 bound to the ubiquitylated nucleosome we explain how specificity is achieved in ubiquitin recognition,” says Dr. Mer.
Expanded knowledge for future therapy
Many important regulatory interactions in the cell rely on recognition of ubiquitin-modified proteins. Structural studies like this one help define how ubiquitin is selectively recognized, as can be seen in the above image.
In this study, published in Molecular Cell, the authors also report that when RNF169, the DNA damage repair protein, binds to the nucleosome, it prevents another DNA repair protein from associating with the nucleosome. That other repair protein, 53BP1, shuts down an alternate pathway to repair DNA double-strand breaks. The authors note that inhibiting this inhibitor could, in principle, be exploited to reactivate this alternate DNA double-strand break pathway in cases where it is defective as in some forms of breast and ovarian cancer.
In addition to Dr. Mer, other authors ─ all from Mayo Clinic ─ are:
- Qi Hu, Ph.D.
- Maria Victoria Botuyan, Ph.D.
- Gaofeng Cui, Ph.D.
- Debiao Zhao, Ph.D.
The authors report no conflict of interest. Access to high field nuclear magnetic resonance instrumentation was provided by the Mayo Clinic Nuclear Magnetic Resonance facility. Funding for this research was provided by the National Institutes of Health, the United States Department of Defense and Mayo Clinic.
###
About Mayo Clinic
Mayo Clinic is a nonprofit organization committed to clinical practice, education and research, providing expert, whole-person care to everyone who needs healing. For more information, visit mayoclinic.org/about-mayo-clinic or newsnetwork.mayoclinic.org.
MEDIA CONTACT
Sara Tiner, Mayo Clinic Public Affairs, 507-284-5005, newsbureau@mayo.edu
Related Articles