-
Cancer
Mayo Clinic researchers discover ‘hidden pocket’ in cancer-promoting enzyme
For years, cancer researchers have been trying to halt a type of molecule that's involved in several cancers. The molecules — enzymes known as trypsins — split proteins that help tumors grow and spread.

Mayo Clinic cancer biologist Evette Radisky, Ph.D., previously found that one trypsin, called mesotrypsin, plays a role in breast, prostate, pancreatic and lung cancer. Like other enzymes, the molecule has an active site that kicks off reactions with other molecules. Researchers have tried to block the active site but haven’t found a molecule with a specific enough lock-and-key fit to jam the active region.
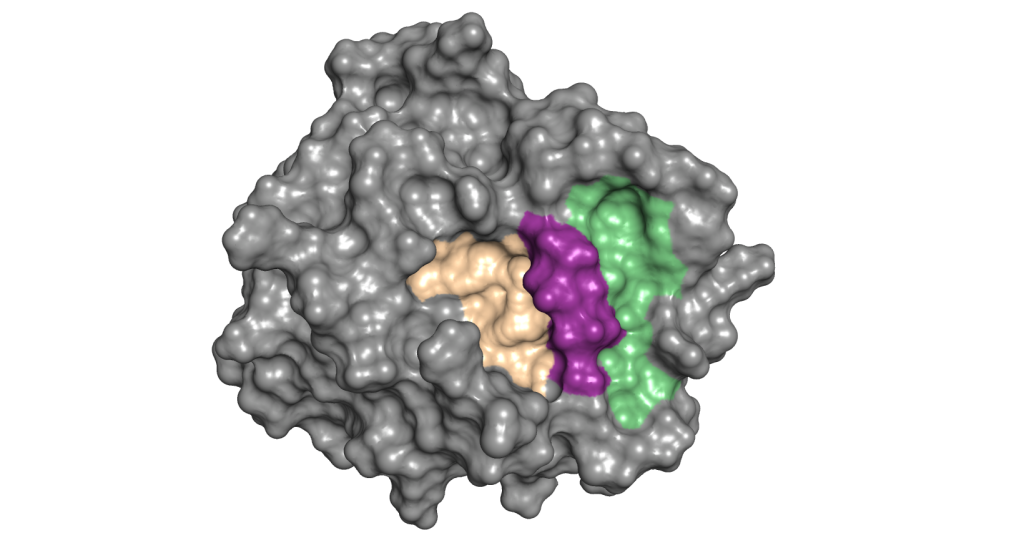
Recently, however, Dr. Radisky's lab at Mayo Clinic in Florida discovered a new way to block mesotrypsin. They found a "hidden pocket" in the molecule.
"The hidden pocket is separate from the active site, but we found that blocking it has a similar effect of locking the enzyme in an inactive state," says Dr. Radisky, principal investigator of the study that appeared in Science Advances. The team now is taking steps to discover drugs that fit the hidden pocket.
A mystery in the data

"It was a serendipitous finding," says the study’s lead author, Mathew Coban, of the pocket's discovery. As a research technologist in the Radisky lab and a master's degree student at Mayo Clinic Graduate School of Biomedical Sciences, Coban had aimed to understand the structure of mesotrypsin through X-ray crystallography.
The complex technique, which records scattered X-rays as shadows, can describe the overall folds of amino acids in the enzyme and suggest complementary molecules that fit like a puzzle. While reviewing the X-ray crystallography results, Coban noticed a segment of the enzyme that looked out of place. The research team thought it might be an error in the data and set the results aside.
But Coban continued to wonder about the strange area. He had the idea to begin looking for alternate nooks in the mesotrypsin enzyme that could potentially contribute to a stable, non-active enzyme.
What Coban found was a site that was hidden. The team dubbed it a "cryptic pocket." The pocket, adjacent to the active site, opened at moments when mesotrypsin stabilized itself. The next step was clear. "If the pocket is there some of the time, maybe a drug would be able to bind at that site and trap the enzyme in its inactive state," he says.
Finding a drug that binds
The team worked with a colleague, Thomas Caulfield, Ph.D., a former Mayo researcher and drug discovery expert, to conduct a computational screen of potential drug compounds that might fit in the cryptic pocket. They found a single molecule that could bind in the cryptic pocket and inhibit the activity of mesotrypsin.
Importantly, the researchers note, the molecule blocks mesotrypsin selectively, without affecting other trypsins. This could mean less toxicity or fewer side effects for a patient. The finding also means that other cryptic pockets may exist in other trypsin molecules related to cancer, presenting new potential drug targets.
The team is continuing to look for drug molecules that fit mesotrypsin even better. "Based on the structural information of mesotrypsin that we have now, we've been able to do more computational prediction to identify additional, more potent compounds that we’re now testing in the laboratory," says Dr. Radisky.
"This has been an important step in the understanding of this key enzyme. Our next steps will be to start testing how well our candidate drug molecules fit the cryptic pocket and block cancer invasion and metastasis in models of disease," she says.
The study was funded by grants from the National Institutes of Health, Mayo Clinic Medical Scientist Training Program and Department of Energy Office of Science User Facility. The authors declare that they have no competing interests.







