-
Mayo Clinic Researchers Identify Enzyme Involved in Deadly Brain Tumors
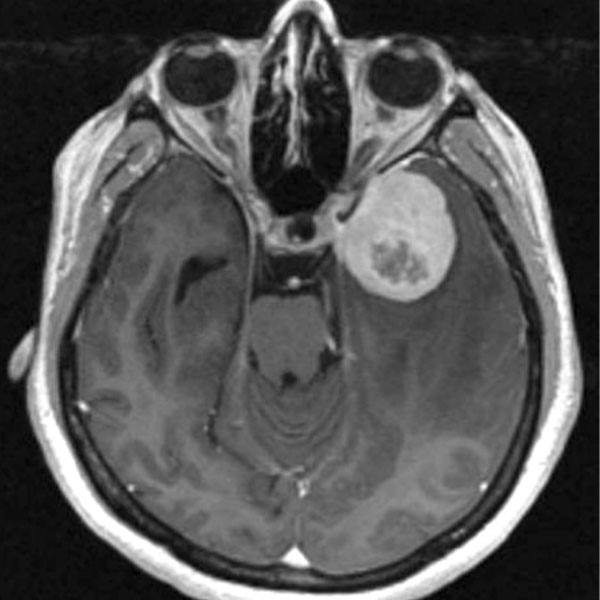
ROCHESTER, Minn. — One of the most common types of brain tumors in adults, glioblastoma multiforme, is one of the most devastating. Even with recent advances in surgery, radiation and chemotherapy, the aggressive and invasive tumors become resistant to treatment, and median survival of patients is only about 15 months. In a study published in Neuro-Oncology, researchers at Mayo Clinic identify an important association between the naturally occurring enzyme Kallikrein 6, also known as KLK6, and the malignant tumors.
"Our study of Kallikrein 6 showed that higher levels of this enzyme in the tumor are negatively associated with patient survival, and that the enzyme functions by promoting the survival of tumor cells," says senior author Isobel Scarisbrick, Ph.D., of Mayo Clinic's Department of Physical Medicine and Rehabilitation.
The findings introduce a new avenue for potential treatment of deadly glioblastomas: targeting the function of KLK6. The tumor cells became more susceptible to treatment when researchers blocked the receptors where the KLK6 enzyme can dock and initiate the survival signal.
Researchers looked at 60 samples of grade IV astrocytomas — also known at this stage as glioblastomas — as well as less aggressive grade III astrocytomas. They found the highest levels of KLK6 were present in the most severe grade IV tumors. Looking at the tumor samples, researchers found higher levels of KLK6 associated with shorter patient survival. Those with the highest levels lived 276 days, and those with lower levels lived 408 days.
"This suggests that the level of KLK6 in the tumor provides a prognosticator of patient survival," Dr. Scarisbrick says.
The group also investigated the mechanism of the enzyme to determine whether it plays a significant role in tumor growth. Researchers also found glioblastoma cells treated in a petri dish with KLK6 become resistant to radiation and chemotherapy treatment.
"Our results show that KLK6 functions like a hormone, activating a signaling cascade within the cell that promotes tumor cell survival," Dr. Scarisbrick says. "The higher the level of the enzyme, the more resistant the tumors are to conventional therapies."
The study is the latest step in Dr. Scarisbrick's investigations of KLK6 in nervous system cells known as astrocytes. Glioblastomas arise from astrocytes that have grown out of control. Her lab has shown that KLK6 also plays a role in the perseverance of inflammatory immune cells that occur in multiple sclerosis and in aberrant survival of T-lymphocyte leukemia cell lines.
"Our findings in glioma affirm KLK6 as part of a fundamental physiological mechanism that's relevant to multiple diseases and have important implications for understanding principles of tissue regeneration," she says. "Targeting KLK6 signaling may be a key to the development of treatments for pathologies in which it is necessary to intervene to regulate cell survival and tissue regeneration in a therapeutic fashion. Ultimately, we might be able to harness the power of KLK6 for the repair of damaged organs."
The study was funded by a National Institutes of Health Brain Tumor SPORE grant, an NIH Mayo Neuro-oncology Training Grant and a grant from the National Institute of Neurological Diseases and Stroke. Other authors include Kristen Drucker, Ph.D., Alex Paulsen, Caterina Giannini, M.D., Ph.D., Paul Decker, Joon Uhm, M.D., Brian O'Neill, M.D., and Robert Jenkins, M.D., Ph.D., all of Mayo Clinic; and Sachiko Blaber and Michael Blaber, Ph.D., of Florida State University.