-
Cancer
Mayo Clinic uncovers hidden driver fueling aggressive prostate cancer
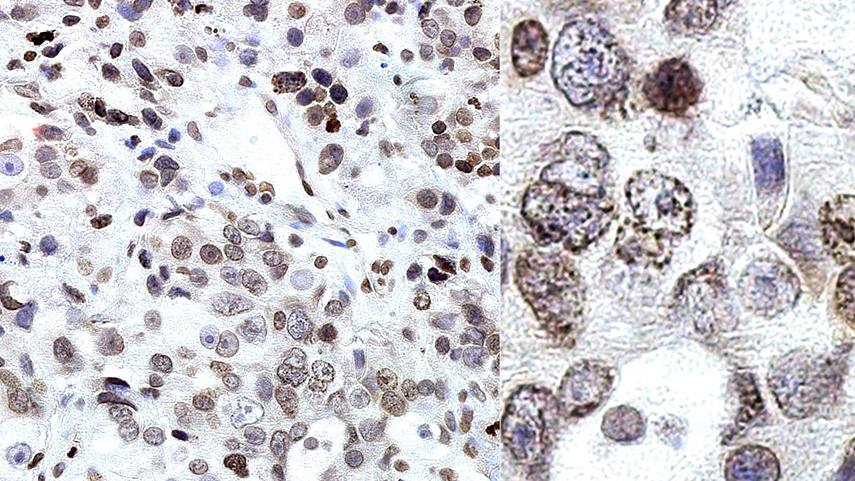
Treatments such as chemotherapy, hormone therapy and immunotherapy have had limited success against advanced prostate cancer due to the tumor's ability to rewire and survive. But hope is on the horizon, thanks to a new Mayo Clinic study that uncovers how prostate cancer exploits a protein in order to resist targeted therapies and evade the immune system.
Nuclear pores are entries to the nucleus made up of proteins called nucleoporins. While nucleoporins usually stay at their post and help regulate the flow of molecules that can come in and out of the nucleus, researchers discovered that soluble POM121 — a subtype of the nucleoporin POM121 — uses its ability to navigate freely to help cancer cells grow and spread.

Dr. Veronica Rodriguez-Bravo, associate professor in the Department of Biochemistry and Molecular Biology at Mayo Clinic Comprehensive Cancer Center and senior author of the study published in Cancer Discovery, says her team's research revealed that soluble POM121, known as sPOM121, builds specialized hubs in the nucleus that rewire gene activity.
"Nobody had looked at the role of this soluble 'off-pore' nucleoporin because in gene expression analyses across databases, everything is classified under the primary nucleoporin. But through detailed analysis we saw an increase in sPOM121 in prostate cancer when compared to noncancerous tissue and a further increase in metastatic therapy resistant tumors," says Dr. Rodriguez-Bravo.
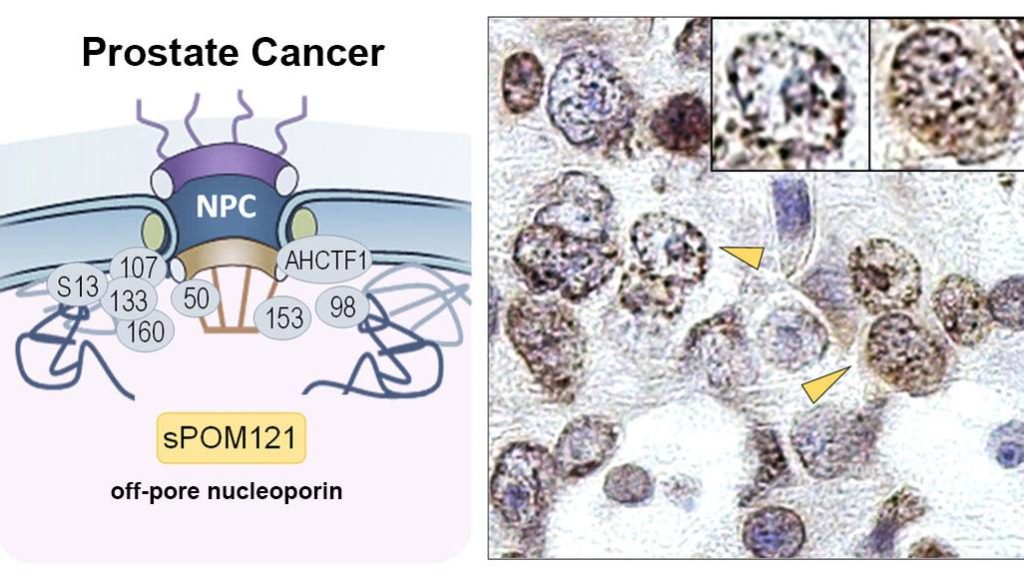
"We started digging for its purpose and found it had a distinctly relevant role in driving prostate cancer progression to lethal stages of the disease," says Dr. Rodriguez-Bravo. The study combined patient tumor analyses, molecular profiling and preclinical models to identify the protein and test its role in metastatic prostate cancer.
The study builds on her previous research, which identified POM121's key role in transporting cancer-promoting proteins into the nucleus.
'Logistic centers' that promote cancer
As a soluble protein, sPOM121 can roam freely in the nucleus, where DNA instructions are stored. "Because it's not attached to the structure that surrounds the nucleus, it can form condensates — which act like logistic centers — and bind to other proteins that regulate gene accessibility and activation," says Dr. Rodriguez-Bravo.
Most notably, sPOM121 partners with a protein called SMARCA5 that modifies DNA packaging to allow a protein called beta-catenin to enhance its expression. Beta-catenin is known to drive therapy resistance and immunosuppressing genes.
Dr. Rodriguez-Bravo says this discovery offers a possible explanation for prostate cancer growth and spread when treated with standard therapy and poor response to immunotherapy.
A potential key to treatment-resistant prostate cancer
The study tested whether blocking sPOM121-driven cancer programs like beta-catenin could improve the effectiveness of treatments such as immune checkpoint inhibitors, which help the immune system attack cancer.
"We found that targeting the soluble POM121-beta-catenin pathway really enhances the effect of immune checkpoint inhibitors. The tumors started getting a lot of T-cell infiltration and shrank," says Dr. Rodriguez-Bravo. "Beta-catenin inhibitors are currently being investigated in multiple tumor types, and our results provide the rationale to investigate the efficacy of these inhibitors alone or in combination with immunotherapy for prostate cancer."
Dr. Rodriguez-Bravo also pinpoints the strong unmet need to develop specific nucleoporin inhibitors that could target multiple cancer-driving signal pathways by disrupting these critical logistic centers. The disruption of these logistic centers by targeting sPOM121 would induce a blackout in the cancer cell, increasing the effectiveness of current therapies, but further investigation is needed to directly target sPOM121, says Dr. Rodriguez-Bravo.
Broader impact beyond prostate cancer
Dr. Rodriguez-Bravo says this study has observed the accumulation of POM121 in multiple tumor types. Her team now hopes to investigate whether the soluble form, sPOM121, is a common cancer driver or plays an earlier role in disease progression.
"If this is really controlling fundamental pathways fueling tumor progression, targeting sPOM121 might potentially benefit more patients in the future," she says.
She also hopes the study will encourage researchers to investigate how cancer might exploit other off-pore nucleoporins.
"We believe sPOM121 is one of many other nucleoporins that orchestrate key transcriptional hubs in the depth of the nucleus. Cancer likes to have a lot of tools on its belt, so we propose these off-pore nucleoporins are selected and used efficiently during tumor evolution," says Dr. Rodriguez-Bravo.
Review the study for a complete list of authors, disclosures and funding.







