-
Discovery Science
More Mayo neuroscience: Brain cells ‘crosstalk’ and cancer growth; Preoperative radiotherapy survival; Rare mutation causes memory loss
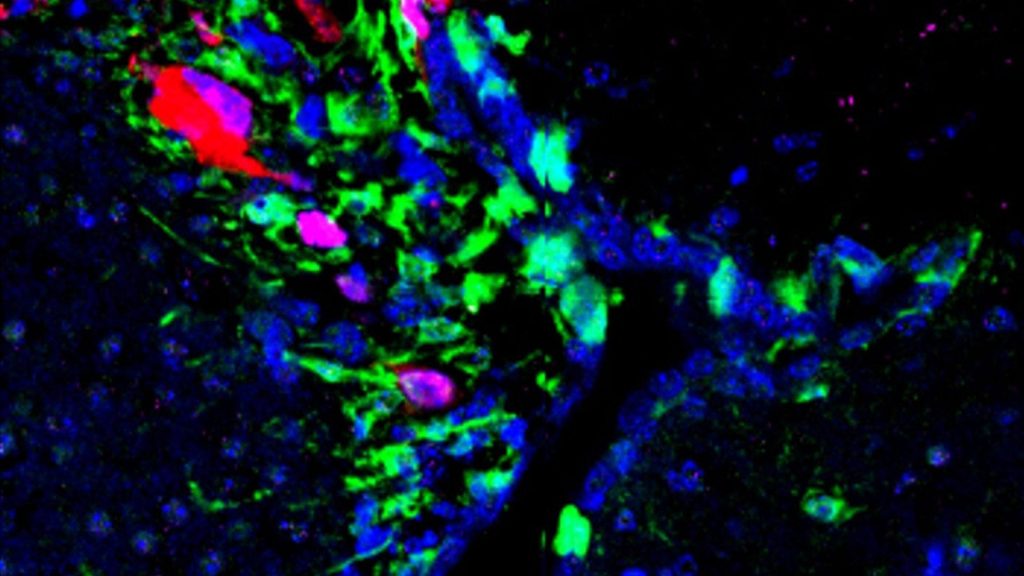
Already the most aggressive form of brain cancer, glioblastoma can become even more deadly when it arises near the brain's C-shaped, fluid-filled cavities called the lateral ventricles.
What makes this type of tumor so aggressive? In a new study published in Science Advances, Mayo Clinic researchers set out to answer this question.
First, the scientists developed a preclinical model in which they fluorescently labeled proteins produced by neural stem cells. With the help of the fluorescence, which colorfully highlights different parts of cells, the scientists could see that glioblastoma-induced senescence, or premature aging, of neural stem cells located in the lateral ventricles, could promote tumor growth.
Next, they looked at glioblastoma cell cultures and discovered that these cells produce elevated amounts of a tumor-promoting protein called cathepsin b (CTSB), which is associated with a worse prognosis for patients.
Through a battery of experiments, the team found that the CTSB protein contributes to increased glioblastoma malignancy. They observed this consistently in cell cultures, animal models and tumor tissue samples — particularly in lateral ventricle-associated tumors — from patients who donated to the Mayo Clinic Neurosurgery BRIDGE Biobank and The Cancer Genome Atlas.
The team hypothesizes that "crosstalk," or communication between neural stem cells and glioblastoma tumors, leads to CTSB protein expression.

"Our findings give us better insight into the biology of glioblastoma, which is essential to finding a treatment or cure for this deadly disease," says senior author Hugo Guerrero Cazares, M.D., Ph.D., associate professor of neurosurgery, cancer biology and neuroscience and leader of the Neurogenesis and Brain Tumors Lab at Mayo Clinic in Florida. Dr. Guerrero Cazares also is vice chair of research in the Neurosurgery Department.
The scientists next plan to build on their findings in two ways: First is identifying proteins or other molecules released by lateral ventricle cells that cause glioblastoma tumors to express CTSB. Second is conducting preclinical studies to target CTSB with pharmaceutical compounds.
Preoperative radiotherapy for glioblastoma enhances survival in a preclinical model
Preoperative radiotherapy is commonly used to treat different cancers that have spread to the brain but has never been used in primary glioblastoma, or cancer that originates in the brain.
Since 2005, the standard of care for glioblastoma has been surgical removal of the tumor, followed by radiation and chemotherapy. Even with this aggressive treatment approach, survival is poor — on average, just under 15 months. This poor outcome is often due to incomplete elimination of the cancer cells, cancer recurrence and the tumor's acquired resistance to therapies. In many patients with newly diagnosed glioblastoma, brain tumors recur immediately after surgery and before chemotherapy is administered.

In a study published in the Journal of Neuro-Oncology, Mayo Clinic researchers found that using a radiation boost before surgery increased survival in a preclinical glioblastoma model, compared to postoperative radiation. The study also sheds light on how this approach impacts the tumor microenvironment—the cellular environment in which a tumor exists—specifically highlighting changes in cell senescence and the movement of cancer-attacking cells to the tumor. The researchers say these insights not only advance the understanding of glioblastoma biology but also propose a pioneering therapeutic approach that could one day benefit patients facing this challenging cancer.
"Our research challenges the conventional treatment sequence and suggests a potentially more effective strategy for managing glioblastoma," says senior study author Paula Schiapparelli, Ph.D., a neurosurgery researcher at Mayo Clinic.
First author Beatriz Fernandez-Gil, Ph.D., says additional laboratory studies and clinical trials are needed to confirm the team's results. Scientists plan to study stereotactic radiosurgery before tumor removal, followed by standard-of-care radiotherapy.
Genetic mutations identified globally for rare disorder causing cognitive decline
A rare cause of hereditary cognitive decline known as CSF1R-Related Disorder (CSF1R-RD) gets its name from mutations in the CSF1R gene. Memory loss occurs as the condition advances, while early symptoms include personality changes, anxiety, depression and loss of inhibition. Genetic testing has become more widely available, but there is no cure for the disorder.
In a new study published in Neurology: Genetics, Mayo Clinic researchers identified eight novel genetic mutations in patients with CSF1R-Related Disorder worldwide. This highlights the prevalence of the disease and paves the way for future individualized treatment. The discovery also suggests that genetic and environmental factors may influence the disease. For example, steroids used to treat inflammation and immune responses can reduce neuroinflammation and prevent symptom occurrence in asymptomatic carriers of CSF1R gene mutations, according to the research team.
Researchers analyzed a range of data — demographics, genotype, family history, clinical status — collected from 14 families from the Americas, Asia, Australia and Europe. They found 15 CSF1R mutations, including eight not reported previously. There are nearly 200 known mutations associated with this disease.

"This study contributes to the overall understanding of the inheritance and global prevalence of rare neurodegenerative conditions in people with and without familial history of the disease," says senior study author Zbigniew Wszolek, M.D., a neurologist and clinical neurophysiologist at Mayo Clinic. "The discovery will allow scientists to target disease-modifying treatments specific to these mutations of the CSF1R gene."
According to Dr. Wszolek, genetic variations can complicate the diagnosis of CSF1R-RD because symptoms can mimic other conditions. He says an accurate diagnosis and medical management of the disease require updated diagnostic criteria and treatment options.
The research team says more studies are needed to look at asymptomatic and symptomatic carriers of the CSF1R gene mutations to better understand the disease. They note that knowledge gleaned from that research will enhance genetic counseling, guide the development of treatment interventions and improve risk prediction for disease onset.
Dr. Wszolek and his research team discovered the CSF1R gene in 2011. One of the team's previous studies on CSF1R-Related Disorder, also known as CSF1R-related leukoencephalopathy, examined a disease-modifying treatment.
Related: Read about other recent neuroscience research breakthroughs at Mayo Clinic.







