-

Preterm Babies Receive Inhaled Nitric Oxide Despite Guidance Discouragement
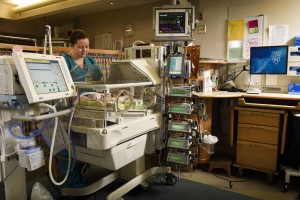 ROCHESTER, Minn. — Inhaled Nitric Oxide (iNO) is a drug approved by the Food and Drug Administration that is commonly used in term and near-term neonates who have severe respiratory failure caused by pulmonary hypertension. Over the last decade there have been multiple large studies trying to determine a clinical use for iNO in preterm neonates, but despite evidence of short-term benefit, this drug has not been shown to improve long-term outcomes in preemies. Still, the drug is commonly being used in this population, Mayo Clinic Children’s Center and co-authors say in a study published today in the journal Pediatrics.
ROCHESTER, Minn. — Inhaled Nitric Oxide (iNO) is a drug approved by the Food and Drug Administration that is commonly used in term and near-term neonates who have severe respiratory failure caused by pulmonary hypertension. Over the last decade there have been multiple large studies trying to determine a clinical use for iNO in preterm neonates, but despite evidence of short-term benefit, this drug has not been shown to improve long-term outcomes in preemies. Still, the drug is commonly being used in this population, Mayo Clinic Children’s Center and co-authors say in a study published today in the journal Pediatrics.
A 2011 statement released by the National Institute of Child Health and Human Development (NICHD) indicated that available evidence did not support the routine use of iNO in preterm neonates and discouraged the use of this expensive therapy in preterm neonates. In 2014, the American Academy of Pediatrics issued a report with similar statements.
MEDIA CONTACT: Kelley Luckstein, Mayo Clinic Public Affairs, 507-284-5005, email: newsbureau@mayo.edu
“Despite professional guidance from the NICHD to discourage off-label iNO use, neonatologists in many NICUs throughout the United States continue to use this medication in the most premature of neonates,” says lead author Marc Ellsworth, M.D., neonatology fellow at the Mayo Clinic Children’s Center.”
Mayo Clinic researchers used data collected by Pediatrix Medical Group, a division of MEDNAX National Medical Group, to identify utilization patterns of iNO in American NICUs in the years surrounding the NICHD statement.
In the population-based study, the researchers found that between 2009 and 2013, the rate of iNO utilization in 23–29 week neonates increased from 5.03 percent to 6.19 percent, a relative increase of 23 percent. Of all neonates who received iNO therapy in 2013, nearly half were less than 34 weeks’ gestation (off label), with these infants accounting for more than half of all first exposure iNO days in each year of the study period.
We now know that off-label iNO use in American NICUs is not decreasing but actually has increased over the last four years, says Dr. Ellsworth. This also has a significant economic effect as well. We estimate that off-label use in the United States generated a cost of $153 million in 2013 alone, he adds.
“It is important that neonatologists discuss with parents fully the possible risks and benefits of this expensive therapy and how it can best be used in their specific child before it is prescribed,” says Dr. Ellsworth.
Co-authors of the study include Malinda Harris, M.D., and William Carey, M.D., of Mayo Clinic Children’s Center; Alan Spitzer, M.D., and Reese Clark, M.D., of Pediatrix Medical Group.
###
About Mayo Clinic
Mayo Clinic is a nonprofit organization committed to medical research and education, and providing expert, whole-person care to everyone who needs healing. For more information, visit mayoclinic.com or newsnetwork.mayoclinic.org.
About Pediatrix Medical Group
Pediatrix Medical Group, a division of MEDNAX National Medical Group, was founded in 1979 and includes neonatal physicians who provide services at more than 370 neonatal intensive care units, and collaborate with affiliated maternal-fetal medicine, pediatric cardiology, pediatric critical care and other physician subspecialists to provide a clinical care continuum. Pediatrix is also the nation's largest provider of newborn hearing screens. Combined, Pediatrix and its affiliated professional corporations employ more than 1,675 neonatal, maternal-fetal and pediatric subspecialists and more than 825 advanced practitioners in 34 states and Puerto Rico. For more information about Pediatrix visit www.pediatrix.com.