-
Arizona
Technology to Help Patients with ALS Provided by Mayo Clinic in Arizona
PHOENIX — Technology to help patients with amyotrophic lateral sclerosis (ALS), commonly known as Lou Gehrig's disease, who experience breathing difficulty is being provided by Mayo Clinic in Arizona.
MULTIMEDIA ALERT: Video of Dr. Ross and Dr. Harold discussing ALS is available for download from the Mayo Clinic News Network.
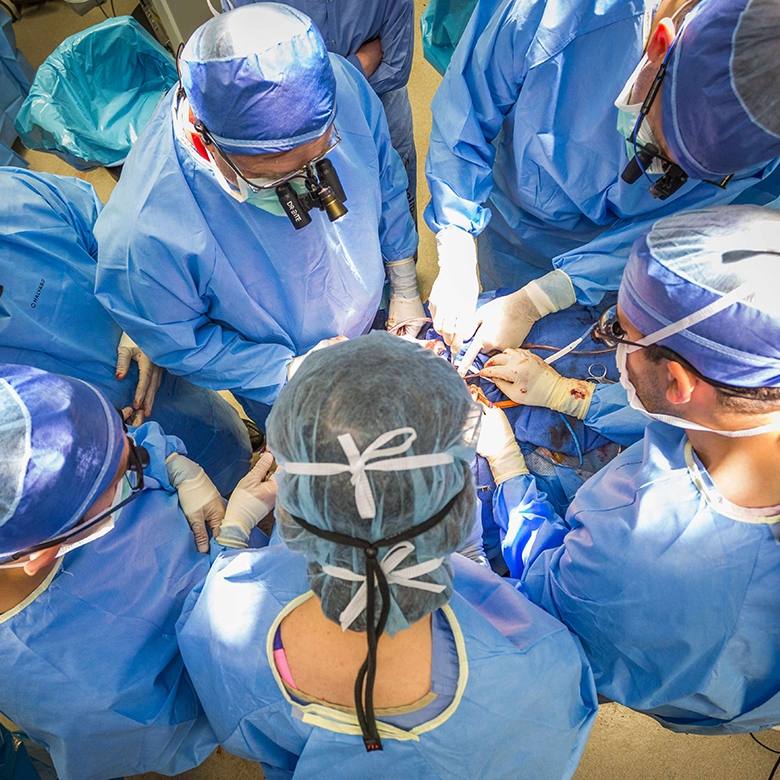
The device, called the NeuRx Diaphragm Pacing System (DPS), is an electronic respiratory assist system that stimulates the nerves in the muscles of the diaphragm. During a minimally invasive surgical procedure, electrodes are implanted into each side of the patient's diaphragm, the body's main breathing muscle. The electrodes connect to a battery-powered stimulator box outside of the body. The stimulator box sends electrical signals to the diaphragm to cause it to contract, thus conditioning the muscles and replicating a natural breathing pattern.
Typically the DPS implantation is a same-day surgery, although some patients may spend a night in the hospital.
The ALS clinic nurse works with the patient to adjust the settings on the external stimulator device. This device is approximately the size of a TV remote, allowing for adjustment of the amount of stimulation to the diaphragm and a comfortable breathing pattern. The system is silent and portable.
Potential benefits identified for patients undergoing the procedure include the ability to delay respiratory decline, provide more comfortable sleep and improve daily quality of life.
DPS is also provided at Mayo Clinic in Rochester, Minn., and Jacksonville, Fla.
ALS affects the motor neurons that signal the muscles used in breathing. During the course of the disease, the diaphragm becomes weakened and many patients experience difficulty breathing, a condition called chronic hypoventilation. When breathing becomes too shallow, many patients require the support of noninvasive breathing devices, which provide pressurized air via a removable face mask. The DPS system is often used in conjunction with non-invasive ventilation.
The NeuRx device was approved by the Food and Drug Administration as a Humanitarian Device Exemption in September 2011.
"We are encouraged about the potential of this therapy to help carefully selected ALS patients with respiratory muscle weakness, says Mark Ross, M.D., Neurology, Mayo Clinic in Arizona. "Because of the electrical stimulation, the diaphragm function may be maintained for a longer time. Patients may experience less breathing problems and will likely live longer because of this device. It is likely that combining DPS and non-invasive ventilation will provide more improvement of respiratory function than either therapy used alone."
The surgery to implant the four small electrodes is performed laparoscopically, through a few small incisions, according to Kristi Harold, M.D., General Surgery, Mayo Clinic in Arizona. "Most patients will undergo programming of the device immediately after surgery and leave the hospital using it to supplement their breathing."
ALS is a devastating neurological disorder with no known cure that affects more than 5,600 people in the U.S. each year, according to the ALS Association. As many as 30,000 Americans have the disease.