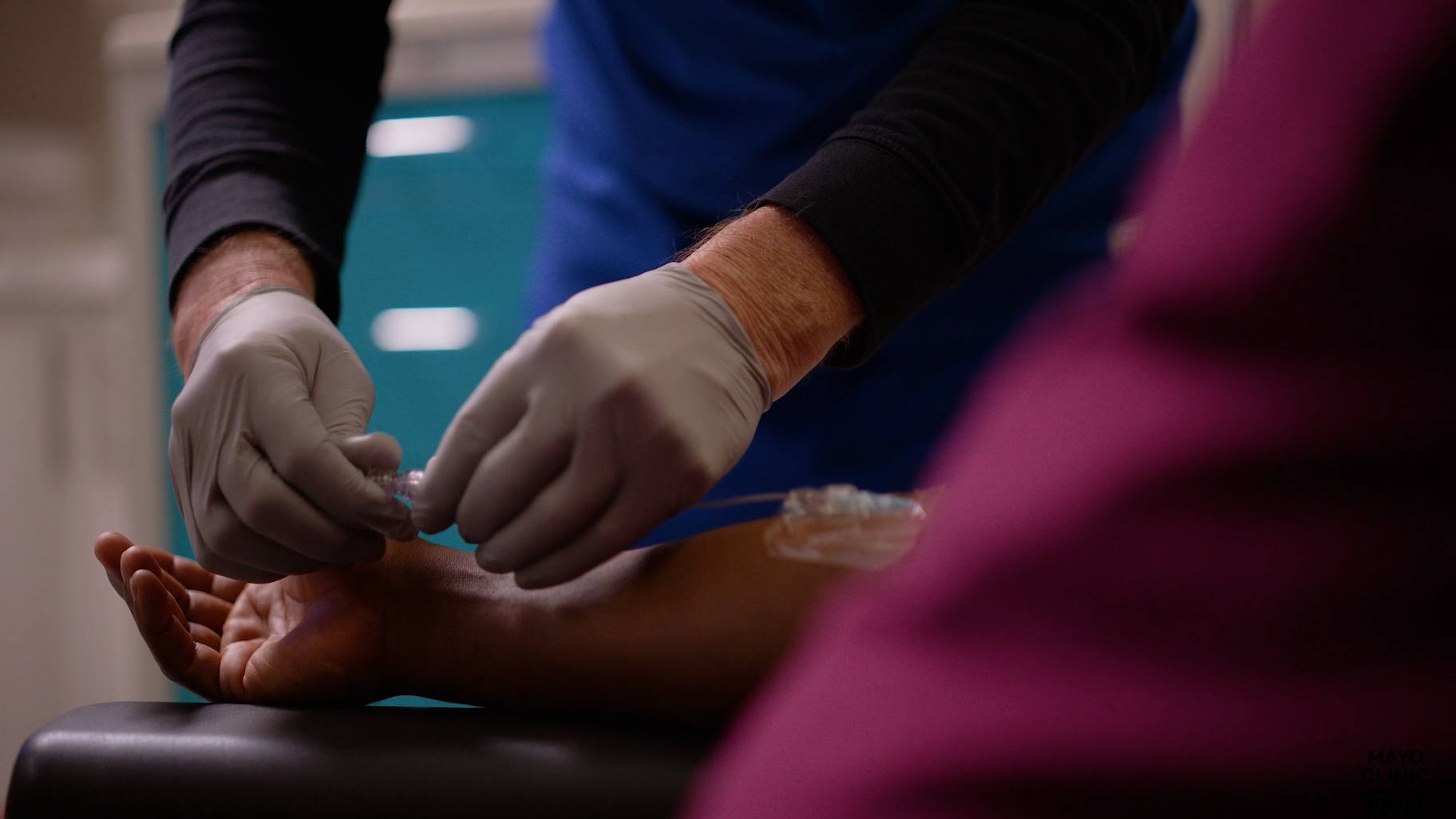
Mayo Clinic is leading the way with a new treatment for advanced prostate cancer, specifically for prostate-specific membrane antigen-positive metastatic castration-resistant prostate cancer (PSMA-positive mCRPC).
This treatment involves a radiopharmaceutical known as Pluvicto. It acts like a smart missile, targeting and eliminating cancer cells.
Mayo Clinic experts offer this therapy for patients whose prostate cancer has spread and is resistant to standard treatments such as hormonal treatments and chemotherapy.
Dr. Oliver Sartor, a Mayo Clinic medical oncologist focused on prostate cancer, says Mayo Clinic is at the forefront of treating patients with this novel therapy, offering new options and hope for patients with advanced prostate cancer. He explains what this means.
Watch: New hope for patients with metastatic prostate cancer
Journalists: Broadcast-quality video (1:19) is in the downloads at the end of this post. Please "Courtesy: Mayo Clinic News Network." Read the script.
Prostate cancer that spreads beyond the prostate is known as stage 4 or metastatic prostate cancer. There's a variety of treatments – including hormonal treatments that can help control the disease.
Therapy for advanced prostate cancer
"Once the hormonal therapies begin to fail, then we have a new term, and we call it castrate-resistant. Metastatic means spread, so it has spread and it's resistant to the hormones. That's metastatic castrate-resistant," says Dr. Sartor.
He says PSMA lutetium-177, the chemical name for Pluvicto, may be an option for some patients.
"We have to do a scan to be able to determine eligibility because we only want to treat the people that have good PSMA uptake. PSMA stands for prostate-specific membrane antigen, and we have a PET scan that can look at that very specifically," Dr. Sartor explains.
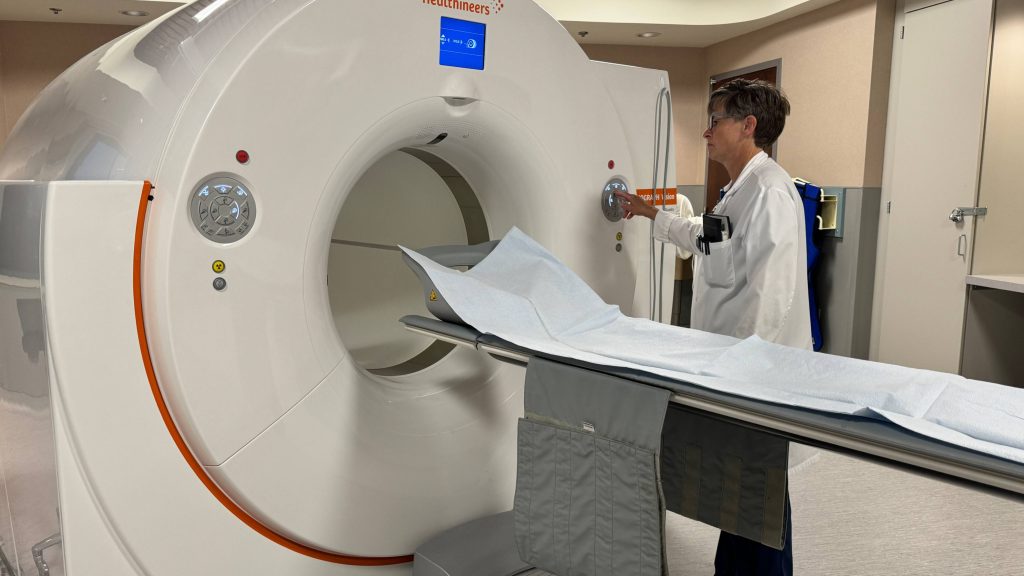
Once eligibility is confirmed, this therapy can be considered.
"It's a radioactive element. It's given intravenously in a short infusion. The FDA-approved regimen is to give it once every six weeks for a maximum of six doses," says Dr. Sartor.
Patients with advanced prostate cancer need a personalized treatment plan. "Please remember that individualization of care is absolutely critical in this disease," he says.
Dr. Sartor is hopeful that this treatment will be made available to patients with prostate cancer earlier in their treatment course.
Conflict of Interest or Disclosure: Dr. Sartor is a paid consultant for Novartis, the maker of the drug Pluvicto.