-
Mayo Clinic: New Mouse Model for ALS and Frontotemporal Dementia Gene Offers Hope for Potential Therapies
JACKSONVILLE, Fla. — Researchers at Mayo Clinic in Florida have developed a mouse model that exhibits the neuropathological and behavioral features associated with the most common genetic form of amyotrophic lateral sclerosis (ALS or Lou Gehrig’s disease) and frontotemporal dementia (FTD), which are caused by a mutation in the C9ORF72 gene.
They say their findings, reported today in Science, will speed further research into the molecular mechanism behind these disorders and that the animal model will offer a way to test potential therapeutic agents to halt the death of neurons in the brain and spinal cord.
MEDIA CONTACT: Kevin Punsky, Mayo Clinic Public Affairs, 904-953-0746, punsky.kevin@mayo.edu
Journalists: Sound bites with Dr. Petrucelli are available in the downloads.
“Our mouse model exhibits the pathologies and symptoms of ALS and FTD seen in patients with the C9ORF72 mutation,” says the study’s senior author, Leonard Petrucelli, Ph.D., Ralph B. and Ruth K. Abrams Professor and chair of the Department of Neuroscience at Mayo Clinic in Florida.
According to the ALS Association, more than 30,000 Americans live with ALS, a condition that destroys motor neuron cells that control essential muscle activity such as speaking, walking, breathing and swallowing. After Alzheimer’s disease, frontotemporal dementia is the most common form of early onset dementia. It is characterized by changes in personality, behavior and language due to loss of neurons in the brain’s frontal lobe. Once considered rare, FTD is now thought to account for up to 10 to 15 percent of all dementia cases, according to the Alzheimer’s Association.
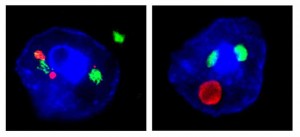
The new mouse model demonstrates that the C9ORF72 repeat expansion, a genetic abnormality discovered in 2011 by Mayo investigator Rosa Rademakers, Ph.D., leads to the generation of toxic ribonucleic acid (RNA) species that form abnormal foci, or clumps, as well as inclusions of c9RAN and TDP-43 proteins. The Petrucelli lab discovered the existence of c9RAN proteins in FTD and ALS cases with the C9ORF72 mutation (c9FTD/ALS) in 2013. However, the new study sheds light on a link between the C9ORF72 repeat expansion and inclusions of TDP-43, a protein that has long been known to go awry in the majority of ALS and FTD cases. TDP-43 inclusions have also been found in Alzheimer’s disease patients and in people who experienced a head injury or repeat concussions.
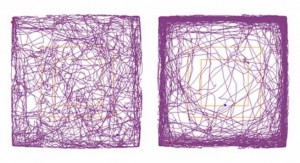
The investigators report these physical and biochemical changes in the mouse brain may be responsible for the observed behavioral deficits and motor impairments akin to human disease symptoms. At six months of age, the mice expressing an expanded C9ORF72 repeat throughout their central nervous system exhibited hyperactivity, anxiety, antisocial behavior and motor deficits.
“Finding TDP-43 in these mice was entirely unexpected — and we could not have discovered a link between the repeat expansion in C9ORF72 and development of TDP-43 pathology without the mouse model,” Dr. Petrucelli says.
“This is a very exciting observation,” he says. “We don’t yet know how foci and c9RAN proteins are linked to TDP-43 abnormalities or what the pathway is, but with our new animal model, we now have a way to find out.”
Through its collaboration with the Scripps Research Institute in Florida, the Petrucelli lab also is working to identify chemical compounds that bind to the repeat-expansion RNA before it can form foci and c9RAN proteins that disrupt nerve cell function. By therapeutically targeting the RNA, thereby preventing the formation of RNA foci and c9RAN proteins in brain cells, the collaborators hope to combat cell death and the associated disease symptoms that c9FTD/ALS patients suffer. Not only will the new mouse model be useful for testing novel chemical compounds, the study findings in the mice suggest that a drug that alleviates the toxicity associated with foci and c9RAN proteins will also prevent TDP-43 pathology.
This work was supported by the National Institutes of Health / National Institute on Aging [P50AG016574]; National Institutes of Health / National Institute of Neurological Disorders and Stroke [R21NS089979, R21NS084528, R21NS079807, R01NS063964, P01NS084974]; Mayo Graduate School; ALS Association; Robert Packard Center for ALS Research at Johns Hopkins; Target ALS; and the Alzheimer’s Association.
Co-authors include first author Jeannie Chew; Tania F. Gendron, Ph.D.; Mercedes Prudencio, Ph.D.; Hiroki Sasaguri, Ph.D.; Yong-Jie Zhang, Ph.D.; Monica Castanedes-Casey; Chris W. Lee, Ph.D.; Karen Jansen-West; Aishe Kurti; Melissa E. Murray, Ph.D.; Kevin F. Bieniek; Peter O. Bauer, M.D. Ph.D.; Ena C. Whitelaw; Linda Rousseau; Jeannette N. Stankowski, Ph.D.; Caroline Stetler; Lillian D. Daughrity; Emilie A. Perkerson; Dr. Rademakers; Dennis W. Dickson, M.D.; John D. Fryer, Ph.D.; Pamela Desaro; Amelia Johnston; Karen Overstreet; Kevin B. Boylan, M.D.; all from Mayo Clinic in Florida. Dieter Edbauer, from the German Center for Neurodegenerative Diseases in Munich, also participated.
Drs. Petrucelli and Gendron have filed a patent (#14/162,570) in the United States regarding the use of poly(GP) immunoassays as a diagnostic for C9ORF72 repeat expansion-related diseases.
###
About Mayo Clinic
Mayo Clinic is a nonprofit organization committed to medical research and education, and providing expert, whole-person care to everyone who needs healing. For more information, visit mayoclinic.com or newsnetwork.mayoclinic.org.







