-
Mayo Clinic researchers identify how fat stores in the liver provide an energy source during fasting
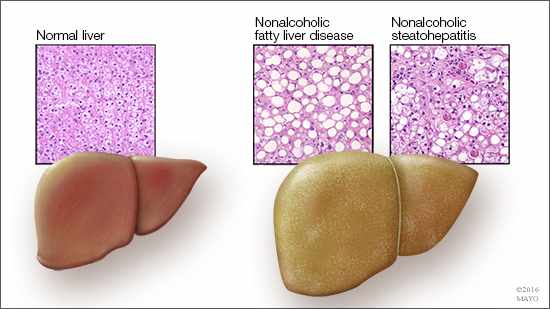 ROCHESTER, Minn. — In a recent Science Advances article, Mayo Clinic researchers show how hungry human liver cells find energy. This study, done in rat and human liver cells, reports on the role of a small regulatory protein that acts like a beacon to help cells locate lipids and provides new information to support the development of therapies for fatty liver disease.
ROCHESTER, Minn. — In a recent Science Advances article, Mayo Clinic researchers show how hungry human liver cells find energy. This study, done in rat and human liver cells, reports on the role of a small regulatory protein that acts like a beacon to help cells locate lipids and provides new information to support the development of therapies for fatty liver disease.
“Between 30 and 40 percent of our population have, or are leading toward getting, nonalcoholic fatty liver disease,” says Mark McNiven, Ph.D., senior author on the paper and director of Mayo Clinic’s Center for Biomedical Discovery. “And that is not including those with alcohol-induced fatty liver; that is also quite prevalent.”
While the mechanisms involved in fat accumulation are the usual targets of research for fatty liver disease, clarifying the cell’s mechanism for breaking down fat also could provide valuable information to fuel the discovery of breakthrough treatments in the future.
Fueling the hungry cell
In a well-fed cell, fat deposits, called lipid droplets, are nutritional insurance. They are ignored by the cell as it fuels growth and division via its normal pathway. But, in a starving cell, the normal pathway switches off, and a recycling process, called autophagy, switches on. Autophagy is a way for cells to break down macromolecules, such as protein and fat, into their component parts to be used in cell processes.
MEDIA CONTACT: Sara Tiner, Mayo Clinic Public Affairs, 507-284-5005, newsbureau@mayo.edu
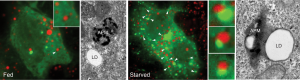
Under starvation conditions, the cell’s recycling pathway directs specialized vessels to engulf lipid droplets. These vessels, called autophagosomes, then link with another organelle, called a lysosome, which is filled with acidic enzymes. When these two merge, the resulting structure is called an autolysosome. Within the autolysosome, the enzymes break apart the fat droplet free fatty acids.
How does a hungry cell find the fat? It follows the beacon
Zhipeng Li, first author and a student at Mayo Clinic Graduate School of Biomedical Sciences, noticed that, within the hungry cells, one protein, called Rab10, was intimately associated with many of the lipid droplets. Rab proteins operate like switches; when bound to a substance, they switch on and facilitate interactions in the cell. There are more than 60 different Rab switches, or small regulatory GTPases, in the human genome.
“In this paper, we show that, when Rab10 is switched on, it will bind to a lipid droplet and cause the autophagosome to dock on the droplet surface, recruit other proteins, and digest the lipid into a free fatty acid energy source,” says Li.
Dr. McNiven explains that cells have sensors that detect low energy levels and respond.
“Rab10 switches on and builds up around the lipid droplet,” says Dr. McNiven. “Then, the cell activates its lysosomes that then targets these lipid droplets and goes after them. So this was an important step that we provided between the sensing mechanism of starvation and how that is signaling to this switch to go after lipid droplets.”
In addition to Dr. McNiven and Li, the other authors on the paper are:
- Ryan Schulze, Ph.D., Mayo Clinic
- Shaun Weller, Mayo Clinic
- Eugene Krueger, Mayo Clinic
- Micah Schott, Ph.D., Mayo Clinic
- Xiaodong Zhang, Ph.D., Mayo Clinic
- Carol Casey, University of Nebraska Medical Center
- Jun Liu, M.D., Ph.D., Mayo Clinic
- Jacqueline Stöckli, University of Sydney
- David James, University of Sydney
This study was funded by two National Institutes of Health grants to Dr. McNiven, the Robert and Arlene Kogod Center on Aging, and the Optical Morphology Core of the Mayo Clinic Center for Cell Signaling in Gastroenterology. The authors report no conflicts of interest.
###
About Mayo Clinic
Mayo Clinic is a nonprofit organization committed to clinical practice, education and research, providing expert, whole-person care to everyone who needs healing. For more information, visit mayoclinic.org/about-mayo-clinic or newsnetwork.mayoclinic.org.
Related Articles







