-
Investigational Oral Drug Combo Shows Promise for Newly Diagnosed Multiple Myeloma in Mayo Clinic-Led Study
ROCHESTER, Minn. — The investigational drug ixazomib taken orally in combination with lenalidomide and dexamethasone shows promise in patients with newly diagnosed multiple myeloma, according to the results of a phase 1/2 study published in the journal Lancet Oncology.
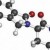
"Ixazomib is an investigational, oral proteasome inhibitor with promising anti-myeloma effects and low rates of peripheral neuropathy," says Shaji Kumar, M.D., a hematologist at Mayo Clinic and lead author of the study. "While it is well known that a combination of bortezomib, lenalidomide and dexamethasone is highly effective in treating newly diagnosed multiple myeloma, we wanted to study the safety, tolerability and activity of ixazomib in combination with lenalidomide and dexamethasone in newly diagnosed multiple myeloma."
Dr. Kumar and colleagues enrolled 65 patients (15 to phase 1 and 50 to phase 2) between November 2010 and February 2012. Researchers established 2.97 mg/m2 as the maximum tolerated dose of ixazomib and recommended the phase 2 dose should be 2.23 mg/m2, which was converted to a 4.0 mg fixed dose based on population pharmacokinetic results. Population pharmacokinetics is the study of the sources of variability in drug concentrations among patients receiving the drug based on demographics, body weight, metabolism and other medications.
There were 41 grade 3 or higher adverse events reported, including skin and subcutaneous tissue disorders neutropenia and thrombocytopenia and drug-related peripheral neuropathy. Five patients discontinued therapy because of adverse events. In 64 response-evaluable patients, 59 (92 percent) had a partial response, including 37 who had a very good partial response or better.
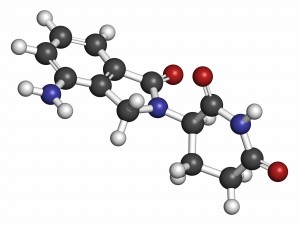 "The all-oral combination of weekly ixazomib plus lenalidomide and dexamethasone was generally well tolerated and appeared active in patients with newly diagnosed multiple myeloma," Dr. Kumar says. "Our results support the development of a phase 3 trial studying this combination for multiple myeloma."
"The all-oral combination of weekly ixazomib plus lenalidomide and dexamethasone was generally well tolerated and appeared active in patients with newly diagnosed multiple myeloma," Dr. Kumar says. "Our results support the development of a phase 3 trial studying this combination for multiple myeloma."
Co-authors include Vincent Rajkumar, M.D., Keith Stewart, M.B., Ch.B., and Vivek Roy, M.D., of Mayo Clinic; Jesus Berdeja, M.D., Sarah Cannon Research Institute; Ruben Niesvizky, M.D., Weill Cornell Medical College; Paul Richardson, M.D., and Jacob Laubach, M.D., of Dana-Farber Cancer Institute; Mehdi Hamadani, M.D., Mary Babb Randolph Cancer Center; Parameswaran Hari, M.D., Medical College of Wisconsin; Robert Vescio, M.D., Samuel Oschin Comprehensive Cancer Institute; Sagar Lonial, M.D., and Jonathan Kaufman, M.D., of Winship Cancer Institute; Deborah Berg, M.S.N., Eileen Liao, Ph.D., Alessandra Di Bacco, Ph.D., Jose Estevam, B.Sc., Neeraj Gupta, Ph.D., and Ai-Min Hui, M.D., of Takeda Pharmaceutical International.
Funding for the study was provided by Millennium Pharmaceuticals, a wholly owned subsidiary of Takeda Pharmaceutical International.
About Mayo Clinic Cancer Center
As a leading institution funded by the National Cancer Institute, Mayo Clinic Cancer Center conducts basic, clinical and population science research, translating discoveries into improved methods for prevention, diagnosis, prognosis and therapy. For information on cancer clinical trials, call 1-855-776-0015 (toll-free).
###
About Mayo Clinic
Mayo Clinic is a nonprofit organization committed to medical research and education, and providing expert, whole-person care to everyone who needs healing. For more information, visit http://www.mayoclinic.org/about-mayo-clinic or https://newsnetwork.mayoclinic.org.
MEDIA CONTACT:
Joe Dangor, Mayo Clinic Public Affairs, 507-284-5005, newsbureau@mayo.edu