Arizona
December 16, 2024
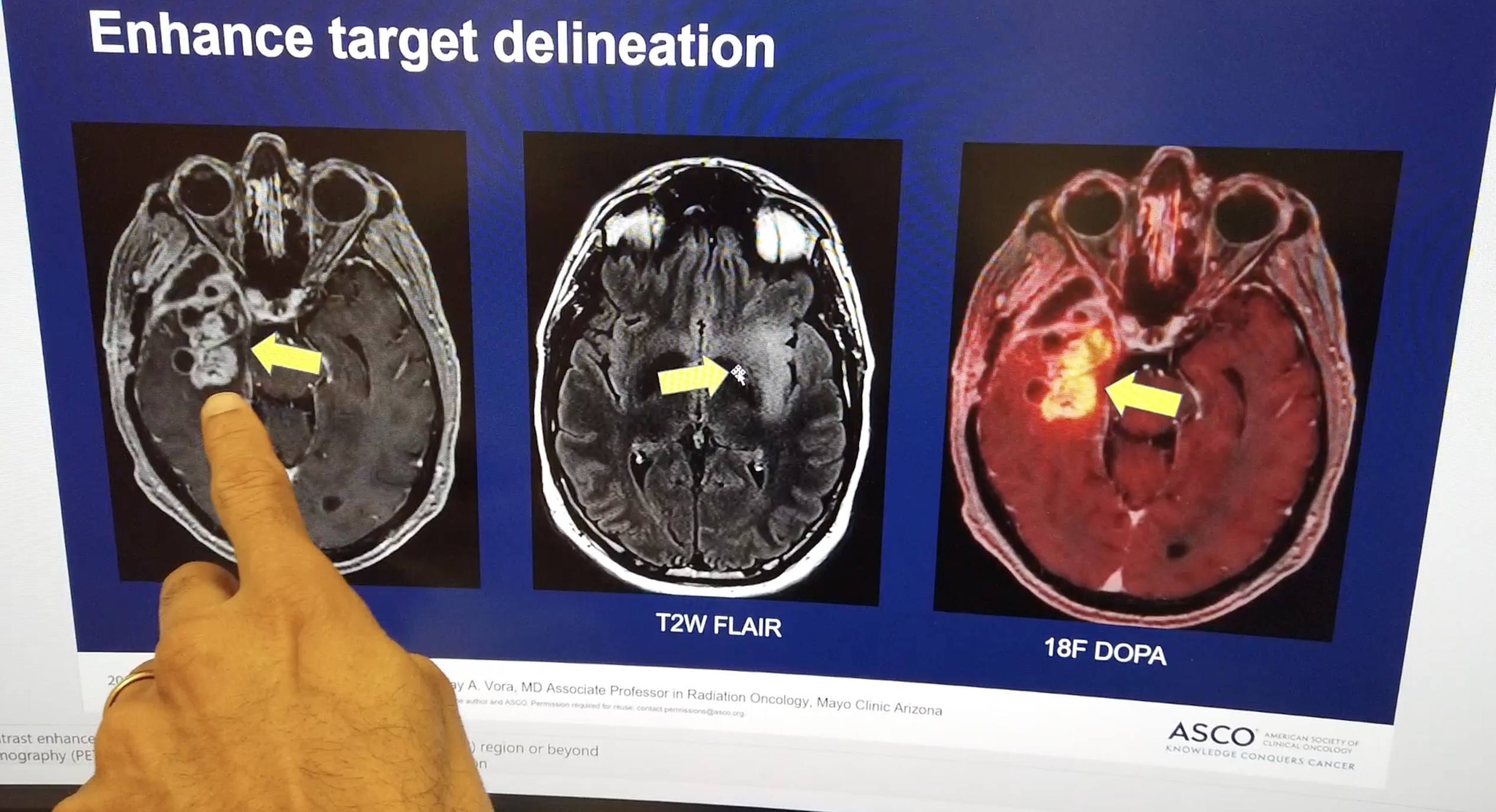
PHOENIX — Mayo Clinic announces the results of an innovative treatment approach that may offer improvement in overall survival in older patients with newly diagnosed[...]
October 21, 2015
Explore more topics
 Sign up
Sign up

Mayo Clinic Connect
An online patient support community


