-
Cancer
Mayo Clinic Offering New “MRI-Safe” Pain-Blocking Device
Mayo Clinic is now offering chronic pain sufferers a new, implantable pain-blocking device approved by the Food and Drug Administration (FDA) — that is safe for full-body MRI, or magnetic resonance imaging, scanners. The device is an advancement on neurostimulation technology that's been is use for decades, but has been denied many patients who would likely need ongoing MRI scans.
Also called spinal cord stimulation, the small, battery-powered transmitters deliver signals through electrical leads implanted along the spinal cord. The signals interfere with pain messages traveling from nerves to the brain. Mayo Clinic pain medicine specialist, Halena Gazelka, M.D., says the devices work extremely well for the majority of people with intractable back, arm and leg pain. But, until now, she's had to tell numerous patients they couldn't use one because MRI scans were more important for managing their medical conditions.
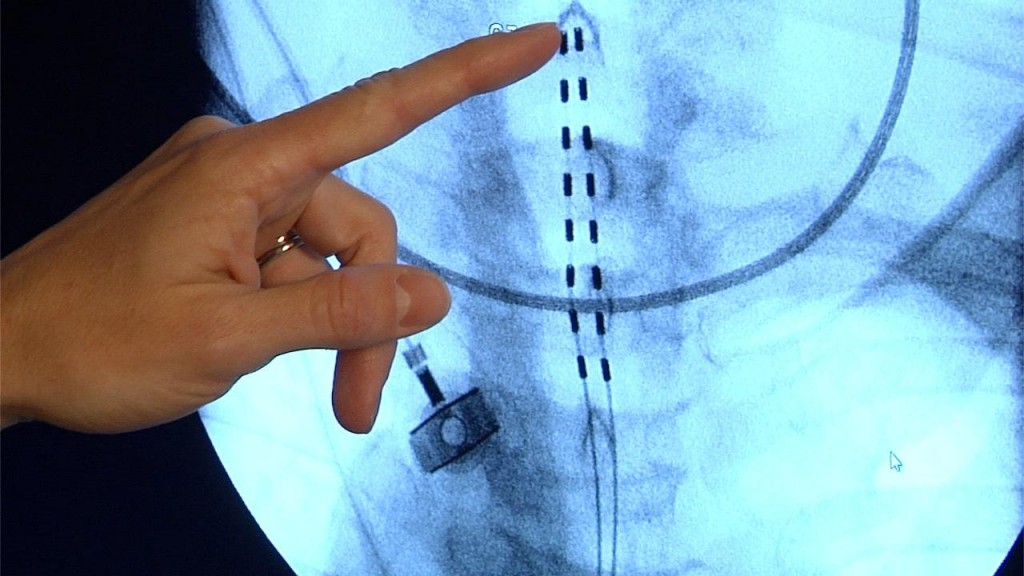
Journalists: Sound bites with Dr. Halena (hah-LEE-nah) Gazelka (gah-ZEL-kah) and b-roll of the device, x-rays and a spinal model are available in the downloads.
Dr. Gazelka says concerns that energy from an MRI scan would heat the impanted materials to the point of causing tissue damage meant that anyone who had one would be barred from future MRIs. But, the newest version, made by Medtronic, is safe because of the way it's made.
/// Sound Bite - WHY IS IT SAFE?: (Dr. Halena Gazelka, M.D., Mayo Clinic Pain Specialist) "There’s a type of coating that they’re placing around the internal portion of the lead that dissipates the energy from the MRI along the entire length of the electrode and lead itself. And then the same thing for the battery pack." [TRT :14]
Dr. Gazelka says she's had success using spinal cord stimulation to help patients manage a variety of conditions, including nerve damage caused by shingles and upper spine pain in the neck. FDA approval for the new Medtronic device is defined for "... use in the treatment of chronic, intractable back and/or limb pain ... for conditionally safe, full-body Magnetic Resonance Imaging (MRI) under specific conditions."
Medtronic says 86.5 percent of clinical trial patients using the new MRI-safe device, the RestoreSensor, reported improved pain relief and/or improved convenience.