-
Untangling the threads of early-onset dementia
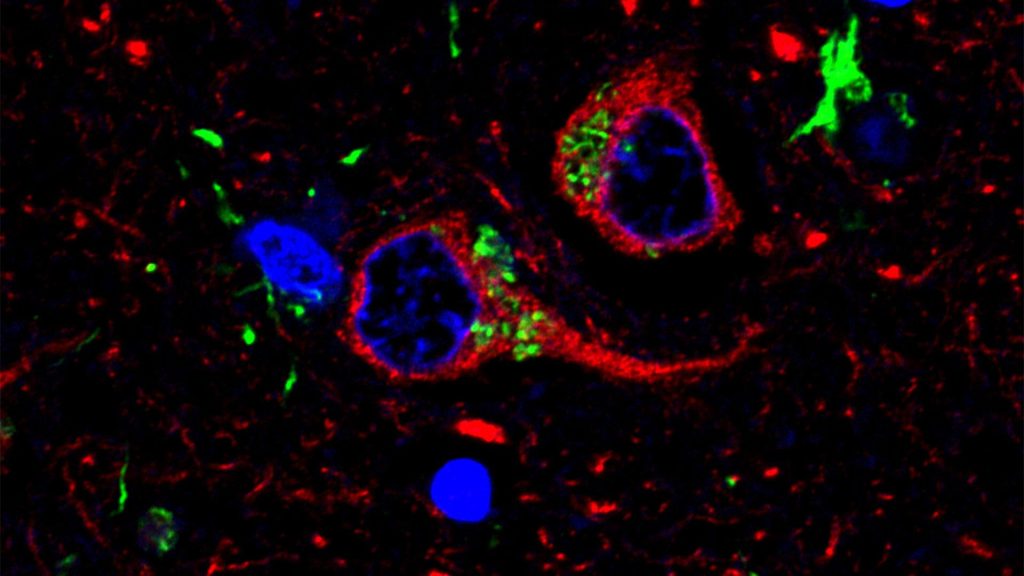
Changes in personality, behavior and language are hallmarks of frontotemporal dementia (FTD), the most common form of dementia in patients under the age of 65, which is associated with degeneration of the frontal and temporal lobes of the brain. Researchers have known that a less common protective variant of a gene called TMEM106B may slow disease progression, and now they have new insight into how parts of the protein produced by the TMEM106B gene may increase risk and cause the disease to accelerate.
They think the key may lie in the formation of fibrils, or tiny fiber-like structures produced by part of this protein, that sometimes get tangled up in the brain through an unknown process. Researchers observed that in most people with FTD whom they studied, these structures pile up, but in those with the protective form, they are virtually absent. The research could pave the way for better treatments in the future.
They report their findings in the journal Science Translational Medicine.
Only recently has the research community discovered that the TMEM106B protein forms these thread-like structures in the brain.
Mayo Clinic researchers in Florida and colleagues set out to determine the link between these TMEM106B structures, the protective TMEM106B genetic variant and FTD. First, they compared disease duration in deceased FTD patients who had donated their brain tissue to the Mayo Clinic Brain Bank. They found that those with the protective variant lived an average of three years longer. This suggests that the disease progressed slower in those patients.
Then, they created an antibody that would allow them to detect the amount of fiber-like structures in the human brain tissue.
Across all FTD cases that researchers analyzed from the brain bank — more than 250 samples — they found that most patients had a relatively high level of these thread-like structures in their brains. However, those with only the protective variant of TMEM106B had little to none. There was a positive correlation between the amount of TMEM106B structures and the level of another pathologic protein called TDP-43, which is strongly associated with FTD.

"It was striking to see that there was no buildup of fibrils in those with the protective variant. We think that likely has something to do with how TMEM106B protects against FTD or alters the disease course, but more work needs to be done to investigate that," says Jordan Marks, an M.D.–Ph.D. student with the Mayo Clinic Graduate School of Biomedical Sciences and first author of the paper. "We also think that these fibrils could one day serve as biomarkers to help clinicians determine FTD prognosis or severity."
The researchers say the findings have implications for future clinical studies.
"Our research provides evidence that genetic variants in TMEM106B are an essential factor to consider in study groups of patients with FTD," says Casey Cook, Ph.D., a Mayo Clinic neuroscientist and co-corresponding author of the paper. "The work also suggests novel therapeutic interventions to prevent the buildup of the tangled fiber-like structures might one day reduce disease risk or slow disease progression."

The next steps in the team's research include validating these results in additional patient study groups and examining the network of interacting proteins associated with FTD to provide further insight into how the TMEM106B protein buildup contributes to disease.
In a related study, also published in Science Translational Medicine, Mayo Clinic researchers and collaborators discovered novel peptides in brain and cerebrospinal fluid produced when TDP-43, also implicated in amyotrophic lateral sclerosis (ALS), or Lou Gehrig's disease, becomes dysfunctional. Their findings could provide the framework for the development of clinical-grade tests to measure TDP-43 pathology in living patients.
Funding for the research on TMEM106B was supported in part by the National Institutes of Health's National Institute on Aging, National Institute of Neurological Disorders and Stroke, Alzheimer's Association, ALLFTD, Cure Alzheimer's Fund, Mayo Clinic Alzheimer's Disease Research Center and Mayo Clinic Foundation. For a full list of authors and disclosures, see the paper.







