-
Aging and Immune Decline: Destiny or Dynamic?
Aging is typically linked with the point in time when it causes problems. But there are many types of aging. There’s chronological age, which we celebrate each year, sometimes with cake; biological aging, which is less celebrated but certainly tracked. Personality and relationships change with age, and aging as a cultural concept affects the way individuals are treated and portrayed. From a medical perspective, aging is a natural process – and better than the alternative. It includes the rise and development of certain functions as well as the decline of others. At a certain point, with biological aging comes an increased risk of dying, most commonly from cancer or chronic illness.
And now, of course, COVID-19.
Some serious health conditions at any age appear to raise a person’s risk of developing severe COVID-19 symptoms. But age, alone, is a risk factor. Even in an otherwise healthy elder, the immune system is — in general — less effective than a younger one. Why this happens still a mystery. It’s tempting to fall back on the general acceptance that, over time, things fall apart, get worn out and work less well. But there are individuals and cultures where chronologic age and biological age have broken out of their parallel paths.

Researchers at Mayo Clinic and around the world are asking, now more than ever, is the link between immune decline and chronological aging our destiny? Or can that risk be lowered by medical intervention? By identifying the hallmarks of biological aging and depicting the ecosystem these cells inhabit, scientists hope their findings will help patients live healthier lives, for longer.
Webs within Webs within Tangles
The immune system, collectively, permeates every nook and cranny in the body. It consists of cells, organs, tissues and protein communication signals that work to keep the body free of infection and disease. There is more complexity to the immune system than contained in any metaphor to describe it, but one of the best, turns back to nature and ecology. The immune system is very much like an ecosystem, rivaling the rainforest in detail, interconnection and change over time; its waterways (lymph channels) and tissue “flora” and white blood cell “fauna” all have the goal of maintaining the integrity of the body.
As an explorer – in this case an immunologist – where would one start? With a survey of the “animals” and their environment.
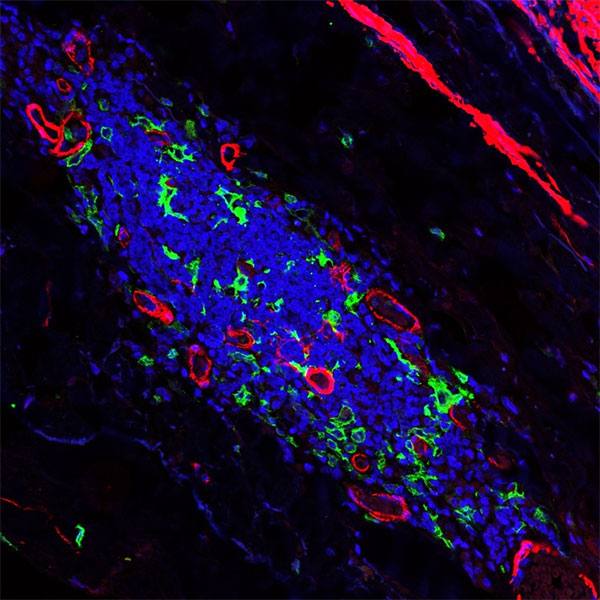
Jessica Lancaster, Ph.D., a Mayo Clinic immunologist, focuses on understanding how cells of the immune system interact and what makes them more effective or less so. She began her career studying the thymus, an organ where T cells “grow up” and that shrinks and becomes less effective as you age. One of her main tools is a microscope that can follow T cells as they move about their ecosystem. T cells are responsible for both organizing immune response and taking direct action to clean up any problems in the body. The supportive cells are ones that gather pieces of foreign material to present to the T cell.

“On the scale that I’m looking at, it looks kind of like the cells are smashing together. The supportive cells, dendritic cells that push T cells into action, are often spidery-shaped. Some have long ‘legs’ if you will, and they’re reaching around in the environment to find the T cell,” says Dr. Lancaster.
In the microscope, Dr. Lancaster and her team use genetic techniques so cells show up as different colors. They can make the supportive cells appear green, for example, and use dyes to clarify the changes in T cells.
“T cells are all labeled red. And they’re kind of circular, but they move like an inchworm. They have a leading edge that sticks out, and they push themselves forward, actively crawling along a kind of a matrix of scaffold cells.”
Using an indicator, such as calcium concentration within T cells, Dr. Lancaster can see the level change and interpret this shift as the T cell has recognized its target and activated. This gives her actual quantitative data, not just an image or video of what's going on inside the tissue environment.
Typically, Dr. Lancaster says, T cells end their travels around the body in the lymph nodes, a part of the lymph system. Similar to an ecosystem’s waterways, the lymph system flows one way. It collects, drains and cleans lymph fluid through a network of tissues and vessels. Seeping in from tissues and the space between cells, the lymph fluid contains, among other things, cells, proteins and antibodies. Lymph nodes are the “pools” along these waterways where white blood cells, such as T cells, prepare to respond to invaders of all sorts or internal threats such as cancer.

“Let’s say you have a virus enter the body. The immune cell that recognizes the virus, takes it in, chews it up and brings it to the closest lymph node. And there the cell waits for a T cell that is able to respond to the chewed up particles.”
In biologically younger people, the areas in the lymph node are correctly compartmentalized, says Dr. Lancaster, meaning that T cells are able to crawl around the structure easily. But as our lymph nodes age, they become disorganized.
“There are inflammatory signals that are probably telling lymph node tissue to deposit collagen, and we end up with a very difficult-to-navigate lymph node. I suspect that this landscape becomes very difficult for T cells to navigate in, and that slows down the ability of a T cell to get activated and go out to direct the immune response.”
Another problem is that the thymus, where T cells mature, becomes smaller as we age. Having a smaller thymus means fewer newly-targeted T cells that can respond to diseases that are new to the body. While the number of T cells remains fairly consistent in the body, T cells that “remember” diseases of the past are most likely to be present and expand to fill the void.
(Read more about how T cells mature over on Advancing the Science.)
“As we age, our T cells become skewed toward producing more memory-type cells, which may have been helpful, if you think of prehistoric man. He's living in the same area, he probably sees the same pathogens over and over again,” explains Dr. Lancaster.

But now, with a global society, increased risks of viral spillover and populations on the move, the modern immune system can face new threats throughout life. To start examining how thymus shrinkage and lymph node disorganization affect the immune response, Dr. Lancaster is focusing on the intersection between these environments and immune cells, specifically how they communicate.
“In older studies, when T cells and B cells from aged individuals were grown in a petri dish and challenged by a pathogen, they actually behaved quite robustly. There's nothing intrinsically wrong with them. But it seems that they're not accessing the correct cues. If you have something that's particularly serious, like COVID-19, where you're rapidly destroying lung cells and your immune response takes another week to mobilize, that could be the difference between life and death for a patient.”
Dr. Lancaster’s goal is to start looking at where communication bottlenecks form, in the hope of finding targets for therapies.
“Then I plan to use the vast collaborative support system at Mayo, where I can talk to people working in drug discovery and people working on the human clinical side. That way, we can create more translational applications to the things that I'm studying in a very basic level.”
From Ecosystem to Interplay
Another researcher investigating the aging immune system is Richard Kennedy, Ph.D., an immunologist and co-director of Mayo’s Vaccine Research Group. Instead of the environment and how it affects immune cells, his team is looking at the system as a whole. They’ve transitioned from looking at one molecule and one effect to a more systematic approach, how all of the interactions between different cells and different molecules and even different locations in the body come into play.

“We’re trying to step back and take more of a systems look at it, and evaluate what is everything doing as we age or as an immune response develops, and how do all of those interactions change,” Dr. Kennedy explains. “So that's orders of magnitude more complex than just looking at a single pathway or a single cell, and while there's some growing pains in moving into that, I think eventually we'll get more information out of this approach.”
The goal of Dr. Kennedy’s lab is to figure out better ways to soften the double whammy of an aging immune system: a slowed response to an infection and a less-robust response to the preventive, a vaccine. “We’d love to figure out why vaccines are less effective, and either create better vaccines or discover a treatment to rejuvenate a person’s immune system,” he says.
But, like any ecosystem, it’s complex. Dr. Kennedy’s lab is looking at symptoms of immune system aging and relating them to key markers in the body. These biomarkers, when combined and examined in a large-enough group of people, could begin to tease out the mechanisms of aging. Each of them, says Dr. Kennedy, catalogues a different piece of what it means to have an “elderly” or “youthful” immune system.
“At a high level, you could break the immune system down into three parts,” he explains.
The first is innate immunity, a nonspecific front line of defense that works broadly against a variety of pathogens. The second part is B cells and antibodies they make. The third part of the system is T cells. There's a variety of types of T cells, but they basically kill off infected cells or guide and direct all of the different cells in your immune system. To add to the complexity of each part, Dr. Kennedy explains that these parts work together but also independently. One still may work while the others are compromised.
One example of immune compromise and biomarkers is HIV/AIDS. The human immunodeficiency virus attacks a type of T cell and turns them into virus factories. The immune system can still provide some protection for the body, but it isn’t as effective as it would be without the leadership of T cells to guide the immune response. A biomarker for HIV might be antigens on HIV, the antibodies made by the body in response to the infection, or the number of CD4 T cells circulating in the body.

Biomarkers for aging immune systems are still very much a puzzle. But in terms of overall changes, Dr. Kennedy’s team is looking at:
- The body’s reduced ability to create new T and B cells
- Increase in pro-inflammatory communication chemicals in the body, leading to high inflammation “background noise”
- Decreased levels of an enzyme that protects chromosomes, called telomerase
- Increased susceptibility to infection
- Senescent, or "zombie," cells that have stopped dividing but refuse the body’s directives to expire.
(For more information on senescent cell biomarkers, read about new research from Mayo, “Zombie Cells: Toxic Ooze Linked to Disease, Disability, Biological Age”)
They’re also tracking changes to specific immune cells (B cells, T cells, macrophages, natural killer), as well as the effect of previous infections. A herpes-like virus called cytomegalovirus (CMV) is one that has particularly piqued the team’s interest.
“Cytomegalovirus infects a lot of people, and, as you get older, your immune system preferentially focuses on that, to the exclusion of other things,” says Dr. Kennedy. “Think of your body as a hotel for memory T cells and B cells. There’s only so much space and resources.”
What the team found is that these CMV-specific T cells and B cells grow to very large numbers and don't work very well. They take up space and resources.
“Now let's say influenza comes along, and you need to mount an immune response,” he continues. “Not only do you have to develop the T cells and B cells specific for influenza, but they've got to push out all of those CMV-specific cells that are hogging all of the space.”
In a clinical trial, the team is examining how aging affects the immune response by studying volunteers age 65 or older. Using two different vaccines for influenza, volunteers will have blood drawn before their particularly assigned vaccine and after vaccination. Then the researchers will bundle the volunteers’ criteria (such as BMI, sex, medications), blood immune cell information, CMV status and markers of immune aging at each blood collection time point.

They are also working with the University of Georgia, a federally designated “Collaborative Influenza Vaccine Innovation Center.” With funding provided by the National Institute of Allergy and Infectious Diseases, the university is working toward a universal influenza vaccine. Their specific focus is to understand the immune response to vulnerable populations: children, pregnant women, people with obesity and the elderly. Dr. Kennedy’s lab is part of that effort as it relates to the aging immune system, and he hopes to expand that work to COVID-19, as well. (Read more in an article from November 2019, on Advancing the Science.)
“The purpose is to take what we've learned from influenza and go back and use existing populations and biospecimens as the pandemic plays out,” says Dr. Kennedy. “At some point in the future, we'll be able to identify people in these cohorts who became infected (with influenza, SARS-CoV-2 or both) and those who did not. Then we'll go back to those biomarkers and ask: 'Is aging playing a role in their susceptibility to becoming infected?'
“We're hoping to drive down into what immune aging really means, and get a better answer than how many birthdays someone has had.”
Conservation, Restoration of the Immune Ecosystem
As immunological-explorers image and trace the web of the immune ecosystem, it’s becoming clear that the terrain of a human's protective bubble begins before birth and grows over time as the body does. The day-to-day choices of eating well, sleeping well and reducing stress have a cumulative effect that is good, but might be much like rain on a parched landscape: good in general, but hit or miss in practice. Dr. Lancaster, as she peers into that complexity, says this knowledge has changed her perspective.
“I'm starting to realize that regardless of your lifestyle, your immune response does change biologically as you get older,” she says. “But I don't think that's fate.”
We should be as healthy as we can, she says, because now there’s a kind of research promise that maybe we can boost those choices so that the 65-year-old marathon dad has as quick a vaccine response as his 33-year-old marathon son.
“There's now this promise that, in addition to making good health decisions, individuals will be able to improve their health span through knowledge gained from aging research, to not just live long lives but healthy lives as well.”
— Sara Tiner, October 20, 2020