Cancer
March 12, 2024
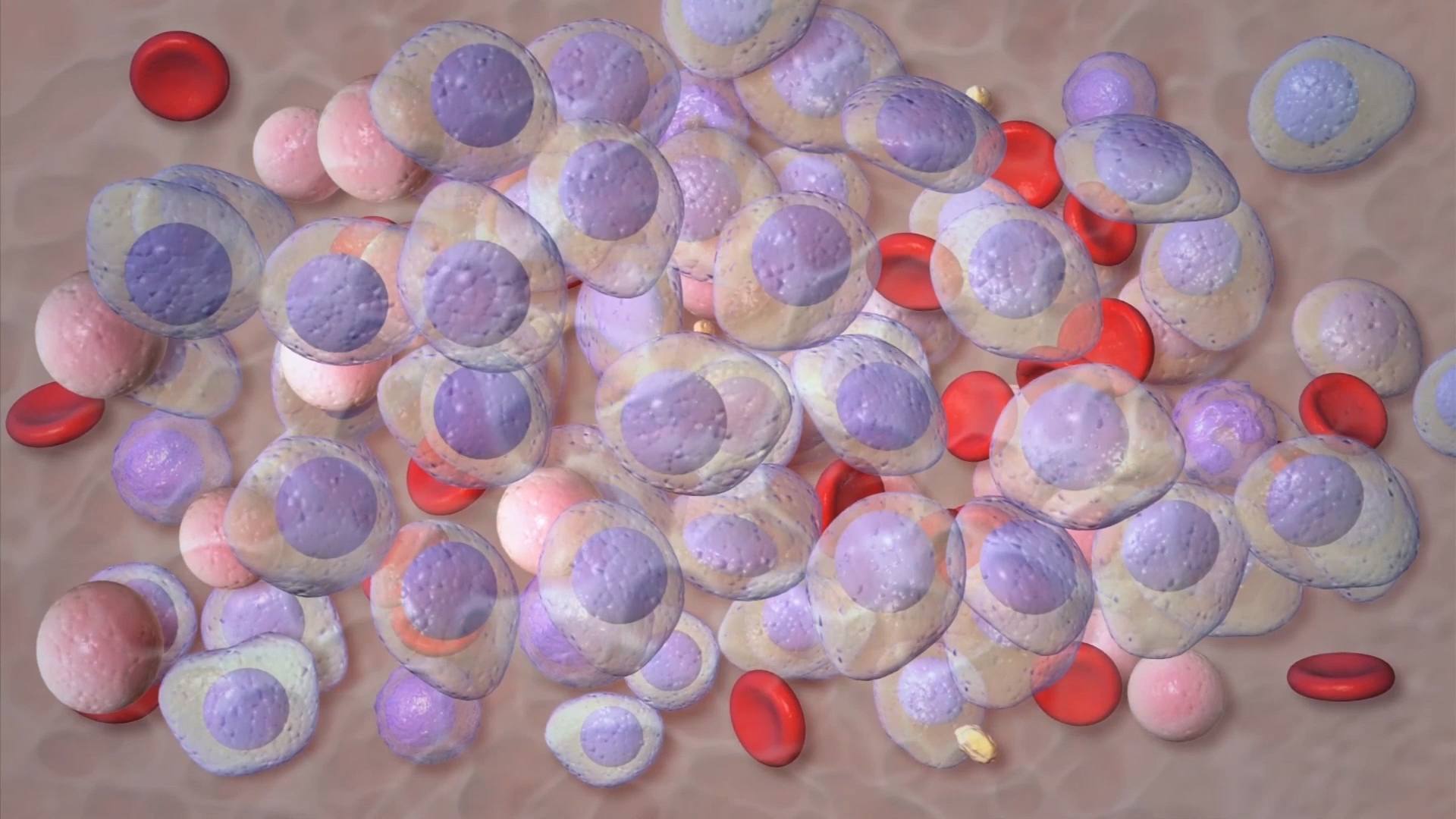
March is Myeloma Awareness Month. Multiple myeloma is a blood cancer that most often occurs in people over age 45. It's the second-most common blood[...]
April 15, 2012
April 11, 2012
April 10, 2012
Explore more topics
 Sign up
Sign up

Mayo Clinic Connect
An online patient support community
