Florida

June 25, 2025
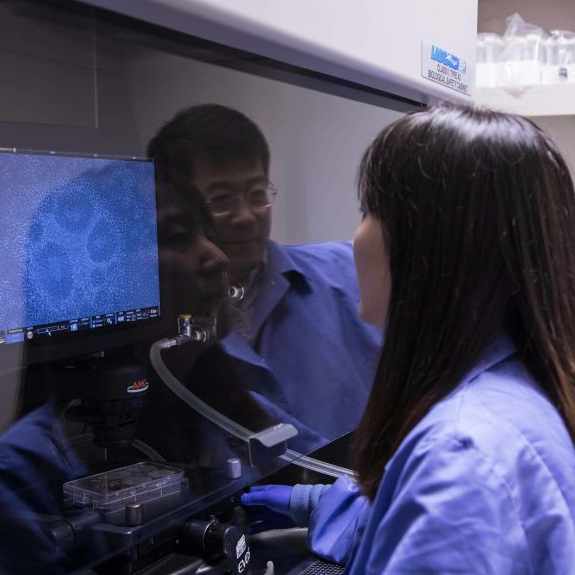
Sue Baker started having issues with her heart in 2015. By 2019, she began experiencing heart failure. Living in Southeast Georgia, her condition landed her[...]

September 18, 2017

September 15, 2017

September 6, 2017

May 31, 2017
Explore more topics
 Sign up
Sign up

Mayo Clinic Connect
An online patient support community